https://meditropics.com/supplement-2022-1/
Rupak Singla Professor & Head, Department of TB & Respiratory Diseases, National Institute Of Tuberculosis & Respiratory Medicine, New Delhi
Epidemiology- Global and India
As per Global TB report 2021, globally in 2020, there were an estimated 157,903 incident cases of drug resistant TB (DR-TB) out of which 132,222 were multidrug resistant/rifampicin resistant MDR/RR-TB and 25 681 cases were of pre extensively drug resistance (pre-XDR-TB) or XDR-TB. India contributes to approximately one fourth of the global burden of TB and MDR-TB. As per India TB report 2020, 2.8% of new cases and 14% of previously treated cases of TB had MDR/RR-TB. (India TB report 2020)
Definitions of various types of drug resistant TB
Multidrug-resistant tuberculosis (MDR-TB): A TB patient, whose biological specimen is resistant to both rifampicin (R) and isoniazid (H) with or without resistance to other first-line anti-TB drugs.
Mono-resistance: A TB patient, whose biological specimen is resistant to one first- line anti-TB drug only.
Rifampicin-resistant tuberculosis (RR-TB): A TB patient, whose biological specimen is resistant to R, detected using phenotypic or genotypic methods, with or without resistance to other anti-TB drugs. It includes any resistance to R, in the form of mono-resistance, poly-resistance, MDR or Extensively drug resistance (XDR).
Poly-resistance: Resistance of the clinical isolate to more than one first line antitubercular drugs other than both isoniazid and rifampicin is referred to as poly-resistance.
Extensively drug resistant TB (XDR-TB): TB caused by Mycobacterium tuberculosis (MTB) strains that fulfil the definition of MDR/RR-TB and are also resistant to any fluoroquinolone (levofloxacin or moxifloxacin) and at least one additional Group A drug (presently to either bedaquiline or linezolid [or both])
Pre-extensively drug resistant TB (Pre-XDR-TB): TB caused by MTB strains that fulfil the definition of MDR/RR-TB and are also resistant to any fluoroquinolone (FQ).
Diagnosis of drug resistance TB
There are two methods available to determine drug resistance/susceptibility:
(I) Genotypic/Molecular testing for resistance: In these tests the information regarding the known gene mutations associated with drug resistance is utilized to diagnose drug resistance. Turnaround time for diagnosis is few hours to 2–3 days only. Commonly available genotypic methods are:
a) Xpert MTB/RIF or cartridge based nucleic acid amplification test (CBNAAT)— This test determines the presence of Mycobacterium tuberculosis and rifampicin resistance gene (rpoB region, which is responsible for approximately 95% of rifampicin resistance in Mycobacterium tuberculosis). The sensitivity of Xpert RIF/MTB has been reported to be high for CSF, lymph node aspirates and gastric aspirates. However, it is low for pleural fluid and other extrapulmonary specimen. Turnaround time for results of Xpert RIF/MTB is 2 hours. Another version, Xpert MTB/RIF ultra, has increased sensitivity to smear positive cases to nearly 100% and to smear negative pulmonary TB to nearly 93%. Xpert MTB/XDR detects mutations associated with resistance towards isoniazid, fluoroquinolones, second-line injectable drug (amikacin, kanamycin, capreomycin) and ethionamide in a single test. Results are available in less than 90 minutes. This test is game changer as it helps in upfront detection of Pre-XDR-TB.
b) Truelab real-time quantitative micro-PCR system (Trunaat)- Truenat MTB and Truenat MTB-Rif Dx (Molbio Diagnostics, Goa, India) are chip-based, micro real-time PCR-based NAAT for TB detection and rifampicin resistance detection, It is based on indigenous technology and can run on battery also.
c) First-Line probe assay (FL-LPA)- LPA version 1 can only be done on samples which are smear positive (direct testing) or on Mycobacterium tuberculosis culture isolates (indirect testing). Turnaround time is up to 72 hours. Its advantage is that besides rifampicin resistance gene rpoB, this test gives information on isoniazid resistance genes katG (high level resistance) and inhA (low level resistance and ethionamide resistance). The disadvantage of LPA is that it is resource intensive and requires trained technicians.
d) Second-line line probe assay (SL-LPA) has been approved by WHO for detecting resistance to 2nd line anti- tubercular drugs – fluoroquinolones and second line injectables. SL-LPA detects mutations in genes gyrA & gyr B for FQ resistance and rrs and eis (low level kanamycin resistance) for SLID resistance. Version 2 of SL-LPA can be used in smear negative cases.
(II) Phenotypic/conventional culture and drug sensitivity testing (DST)-They can be done on either
a) Solid culture—It is done on Lowenstein–Jensen and Middlebrook 7H10/11. The turnaround time for solid culture is up to 42 days and for DST is up to another 42 days.
b) Liquid culture (LC)—Also called as BACTEC, MGIT960 (BD Diagnostics) and BacT/ALERT The turnaround time for liquid culture and DST is usually up to 3 – 4 weeks. MGIT is the preferred method for DST because of reduced turnaround time. It can be used for testing both the pulmonary & extra-pulmonary specimens for sensitivity to first as well as second-line anti-TB drugs. Liquid culture is also used to monitor response to treatment and for long-term follow-up of patients on drug resistant TB treatment.
Integrated drug-resistant TB algorithm

Upfront NAAT will be offered to presumptive TB patients in key populations, all diagnosed TB patients and treatment non-responders. Key populations include PLHIV, children, extrapulmonary TB (EP-TB), smear negative/not available (NA) with chest x-ray suggestive of TB, contacts of DR-TB patients and other vulnerable groups. All TB patients in whom an appropriate specimen can be collected are to be offered NAAT for bacteriological confirmation of TB and RR-TB & further test for fluoroquinolone (FQ) by SL-LPA.
The algorithm helps to segregate the patients based on NAAT results as Rifampicin resistance (RR) detected or RR not detected and offer DST guided treatment.
-The left arm: Rifampicin resistance detected When rifampicin resistance is detected, the patient is offered first-line (FL) and SL-LPA. RR detected in a new case with no risk factors for DR-TB needs to be retested if only M. tb detected was very low as that could be false positive. While FL LPA provides information on mutations associated with isoniazid resistance, SL LPA provides information on resistance to Lfx, Mfx and Am. LC DST would be set up for Pyrizinamde (Z), Moxifloxacin (Mfx) (if resistance detected by LPA), Linezolid (Lzd), Clofazimine (Cfz)*, Bedaquiline (Bdq)* and (delamanid) Dlm* (* when available). Treatment is initiated based on the results of FL-LPA and SL-LPA and if required modified based on the LC DST results which would be available later. If FQ resistance is not detected and H resistance is detected due to mutations either in katG or InhA (but not both) the patient is eligible for shorter MDR/ RR-TB regimen. If FQ resistance is detected or H resistance is due to mutations in both katG and InhA, the patient is eligible for longer oral M/XDR-TB regimen.
-The right arm: Rifampicin resistance not detected When rifampicin resistance is not detected, the patient is offered FL LPA for detecting resistance to H. If H resistance is not detected, the patient is continued on a Drug-sensitive TB regimen. If H resistance is detected, the patient is eligible for H mono/Poly DR-TB regimen. SL LPA will be performed for detecting resistance to FQ and LC DST will be performed for Mfx (if resistant by SL LPA), Z, Lzd and Cfz* (*when available). Treatment is initiated based on LPA results and modified based on the LC DST results which would be available later.
If there is a discordance in rifampicin resistance between NAAT and LPA, a second NAAT is performed at the Culture & DST laboratory using the decontaminated deposit and microbiologist will provide the final decision. Direct LPA can be performed only on smear positive specimen. In instances where the smear is negative, a culture is set up and if the culture is positive, LPA is performed on the isolate.
Management of DR-TB
Newer Drugs
Bedaquiline (Bdq) is a diarylquinoline that specifically targets mycobacterial ATP synthase, an enzyme essential for the supply of energy to Mycobacterium TB. Pre-clinical, laboratory and animal experiments have shown strong bactericidal and sterilizing activities against M.tb. As per PMDT 2021 guidelines Bedaquiline has been approved for children 5 years of age and above and weighing ≥ 15 Kgs.
Delamanid (Dlm) is the first approved drug in the class of nitro-dihydro-imidazo-oxazoles for the treatment of MDR-TB. It is bactericidal drug. Dlm is already approved for use and available under NTEP from 6 years onwards.
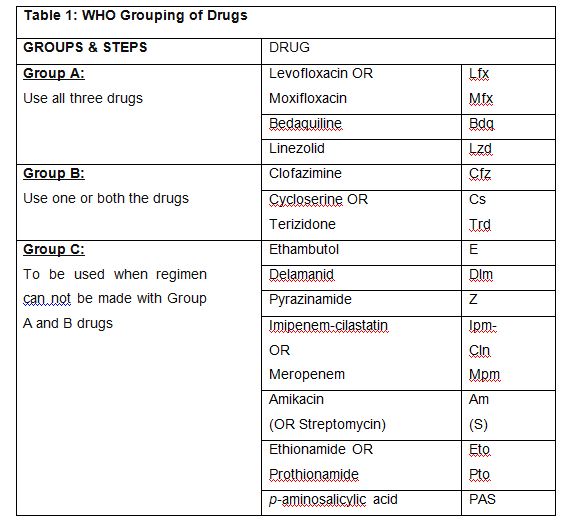
Table 1: WHO grouping of anti-TB drugs used for longer m/XDR-TB regimen
Regimen for DR-TB treatment
The standardized treatment regimen for M/XDR-TB include:
- All oral H mono/poly DR-TB regimen
- Shorter RR/MDR-TB regimen which could be Bedaquiline containing oral regimen or injection containing regimen
- Longer oral regimen
Table 2: Standard regimen for initiating treatment of MDR/RR TB or H mono-poly DR TB

*If the intensive phase is prolonged, the injectable agent is only given three times a week in the extended intensive phase.
# Reduce Lzd to 300 mg/day after 6 to 8 months.
@ Pyridoxine to be given to all DR TB patients as per weight band.
All oral H mono/poly DR TB regimen is of 6 months with no separate IP/CP. Shorter injection containing MDR TB regimen is of 9-11 months with 4-6 months of IP containing injectables and 5 months of CP. If the IP is prolonged, the injectable is only given three times a week in the extended intensive phase. Shorter oral MDR TB regimen consists of an initial phase of 4 months that may be extended up to 6 months and a continuation phase of 5 months, giving a total duration of 9–11 months. Bdq is used for a duration of 6 months. IP should be given for at least 4 months.
^ Longer oral regimen: Longer oral M/XDR-TB regimen is of 18-20 months with no separate IP or CP. The composition of this regimen is bedaquiline (6 months or longer), levofloxacin, linezolid*, clofazimine, cycloserine (*linezolid dose is to be reduced after 6-8 months). In XDR-TB, the treatment is to be given for 20 months.
Inclusion and Exclusion criteria for shorter MDR regimen
Inclusion criteria
DST based inclusion criteria
- Rifampicin resistance detected/inferred
- MDR/RR-TB with H resistance detected/inferred based on InhA mutation only or based on KatG mutation only (not both)
- MDR/RR-TB with FQ resistance not detected
- Other inclusion criteria
- Children, aged 5 years to less than 18 years of age and weighing at least 15 kg, given their special needs, in consultation with the paediatrician
- No history of exposure to previous treatment with second-line medicines in the regimen (Bdq, Lfx, Eto or Cfz) for more than 1 month (unless susceptibility to these medicines is confirmed)
- No extensive TB disease
- No severe extra-pulmonary TB
- Women who are not pregnant or lactating
Exclusion criteria
- DST based exclusion criteria
MDR/RR-TB patients with H resistance detected with both KatG and InhA mutation; and
MDR/RR-TB patients with FQ resistance detected.
- Other exclusion criteria
- If result for FL-LPA, SL-LPA and DST to Z, BDQ* & Cfz* is not available after pre-treatment
- evaluation is completed and it is a time to initiate the first dose of the regimen, then, exclude those with history of exposure for > 1 month to Bdq, Lfx, Eto or Cfz;
- Intolerance to any drug or risk of toxicity from a drug in shorter oral Bedaquiline containing MDR/RR-TB regimen (e.g., drug–drug interactions);
- Extensive TB disease found in presence of bilateral cavitary disease or extensive
parenchymal damage on chest radiography. In children aged under 15 years, presence of cavities or bilateral disease on chest radiography;
- Severe EP-TB disease where there is a presence of miliary TB or TB meningitis or central nervous system (CNS) TB. In children aged under 15 years, extrapulmonary forms of disease other than lymphadenopathy (peripheral nodes or isolated mediastinal mass without compression);
- Pregnant and lactating women and Children below 5 years
Principles of Designing an a WHO recommended all oral longer MDR-TB regimen
Whenever an MDR-TB regimen is being designed the following principles should be kept in mind:
- MDR-TB regimen should be composed of at least 4-5 drugs likely to be effective, including all drugs of group A plus 1-2 drugs from group B. Drugs from group C can be used if the ones from group A or B is contraindicated or not tolerated, so as to complete the regimen. WHO recommended to start treatment with all three Group A agents and at least one Group B agent to ensure that treatment starts with at least four TB agents likely to be effective and that at least three agents are included for rest of the treatment if Bdq is stopped. However, in India the experts concurred to start with all 5 drugs of Group A and B and continue with 4 drugs in the latter part of the regimen (beyond 6-8 months) if the patient can tolerate the drugs and for operational ease in the field.
In case of the need for replacement of any of the component(s) in the longer oral M/XDR-TB regimen, the following broad principles apply:
The drugs are replaced according to their efficacy, no demonstrable resistance, prior use, side-effects profile and background resistance to the replacement drug in the country as per the National Drug Resistance Surveillance report. The regimen should preferably be fully oral. However, in certain circumstances, injectables may have to be used for the need of efficacy and side-effect profile. In situations where no replacement of any drug is required in the first 6 or 8 months of treatment in MDR-TB or XDR-TB patients, continue with at least 3 drugs after this depending upon resistance, tolerability, availability, contraindication etc. of any one of Group A or B drugs. As per PMDT guidelines 2021, the replacement sequence of Group C drugs for longer oral M/XDR-TB regimen is recommended in the order of – delamanid, amikacin, pyrazinamide, ethionamide, PAS, ethambutol, penems. Dlm and Am will not be started in the final 12 months of treatment.
Drug dosage and administration for Drug Resistant TB
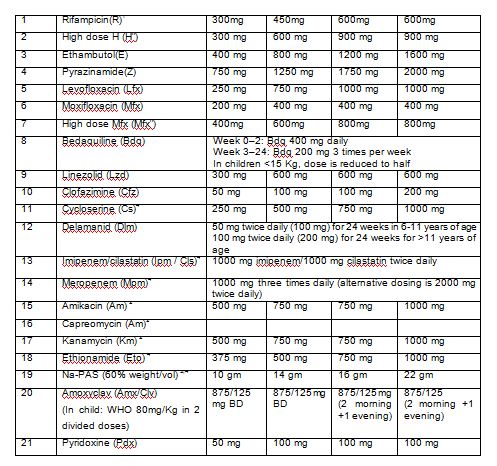
The dosage of drugs varies as per weight of the patients. Adult patients (≥ 18 years) are classified in weight bands of 16-29 kg, 30-45 kg, 46-70 kg and >70kg. The dosage for drugs used in various DR TB regimens by weight bands for adults are enumerated in Table 3.
Table 3: Dosage of DR TB drugs for adults
BPaL regimen
Treatment regimen lasting 6–9 months, composed of bedaquiline, pretomanid and linezolid (BPaL). It is under operational research conditions, and thus does not apply to routine programmatic use. It is to be used in multidrug-resistant tuberculosis (MDR-TB) patients with TB that is resistant to fluoroquinolones, and have either had no previous exposure to bedaquiline and linezolid or have been exposed for no more than 2 weeks.
Role of Surgery
Lung resection with adequate chemotherapy may be helpful in carefully selected patients (localized disease) in centers with adequate resources and expertise, as this may help in getting rid of the intractably pathological part and reduce the bacterial load needed to be eliminated. Adjunct resection surgery may also reduce the chances of relapses.
Conclusion
- Focus on Prevention of DR-TB by ensuring that patient takes full treatment with adequate dosages regularly throughout the course.
- Phenotypic tests take longer time, but good to assess response to treatment.
- Genotypic tests are faster and help in early initiation of appropriate regimen.
- Newer Genotypic tests such as Xpert MTB/XDR maybe game changer for early identification of Pre XDR-TB and early initiation of appropriate regimen.
- Follow Integrated diagnostic and treatment algorithm for management of DR-TB patients.
- New drugs- Bedaquiline and Delamanid are promising and should be used wherever indicated.MANAGEMENT OF MDR-TB