Pulse pressure and Electrocardiographic Parameters like QTc, QTd : Easiest Marker of Cardiac Autonomic Neuropathy and Determinants of further Complications of Type 2 Diabetes Mellitus
Rajesh K Meena 1
- Department of Internal Medicine, Lady Hardinge Medical College New Delhi, India
https://meditropics.com/original-article-2022-2/
Abstract:
Background: To determine the profile of pulse pressure, corrected QT interval and QT dispersion in Type 2Diabetes Mellitus, and also study the relation between pulse pressure, corrected QT interval and QT dispersion with glycaemic status and insulin resistance
Methods: This observational study was conducted in the Department of Medicine Lady Hardinge Medical College New Delhi. 160 types -II diabetic mellitus above 35 age group patients are enrolled. Diagnosed case of T2 DM less than 5 years and newly diagnosed cases as per ADA 2015 criteria are recruited. Detailed history & examination (including anthropometry and thorough systemic examination) of all the cases was done & data collected was entered in a pre-designed Performa and subjects was called on another date after 8 hours of overnight fasting for biochemical and pathological tests. Other investigation like 12 lead ECG with rhythm strips, urinary tests were also carried out on the day of next visit.
Results: showing average age of subjects included was 52.01±8.39 years, mean duration was 2.47±1.55 years, mean BMI was 26.13±3.4, mean WC was 93.86±7.24 cm, mean PP was 50.44±12.03 mmHg, mean FBG was 159.69±40.93 mg/dl, HbA1c was 8.29%±1.84%, average HOMA-IR was 4.26±1.76, mean QTc interval was 422.52±41.07 and mean QTd interval was 58.19±32.43. showing 55% study subjects were male, 72.51% subjects were in age group of 41-60years, 70% subjects were in obese category, prevalence of wide PP was 31.30%, QTc prolongation was 33.75% and QTd was 37.50%. Abnormal HOMA-IR was in 70.63% study subjects. The prevalence of microvascular complications like retinopathy, nephropathy and neuropathy were 30.63%, 25.63% and 18.13% respectively.
Pulse Pressure (50.44±12.03), QTc interval (422.52±41.07) and QTd interval (58.19±32.43) were quite prevalent 31.30%, 33.75% and 37.50% respectively in type 2 diabetes. The prevalence of microvascular complications like retinopathy, nephropathy and neuropathy were 30.63% (n=49), 25.63% (n=41) and 18.13% (n=29) respectively. Present study revealed that PP was associated with age, HOMA-IR and retinopathy, a significant difference (p=0.0002), (p=0.0003) and (p=0.0003) were also found with age, HOMA-IR and retinopathy respectively in subjects with normal and wide PP.
Conclusion –
Present study revealed that PP was associated with age, HOMA-IR and retinopathy while QTc interval was associated with HOMA-IR, retinopathy and nephropathy. Also, QTd interval was associated with age, HOMA-IR, retinopathy, nephropathy and neuropathy.
Key words: Diabetes, pulse pressure, QTc interval , QTd , HbA1c , Insulin Resistance , Cardiac autonomic neuropathy
Aims & Background:
According to the latest 2016 data from the World Health Organization (WHO) an estimated 422 million adults are living with diabetes mellitus. The number is projected to almost double by 20301. Type 2 diabetes makes up about 85-90% of all cases. The underlying metabolic causes of T2DM are the combination of impairment in insulin mediated glucose disposal (insulin resistance) and defective secretion of insulin by pancreatic beta cells. Insulin resistance develops from obesity and physical inactivity, acting on a substrate of genetic susceptibility. Insulin secretion declines with advancing age2, and this decline may be accelerated by genetic factors3.
Insulin resistance typically precedes the onset of type 2 diabetes and is commonly accompanied by other cardiovascular risk factors: dyslipidemia, hypertension and pro-thrombotic factors 4. The common clustering of these risk factors in a single individual has been called the metabolic syndrome.
Patients with T2DM are at increased risk of dying from cardiovascular diseases. Excess cardiovascular risk in this population persists even after normalization for other cardiovascular risk factors (hypertension, dyslipidemia, physical inactivity, smoking), suggesting that there are other incompletely understood mechanism which increases cardiovascular risk in diabetic patients.
Autonomic neuropathy is a well-recognized complication of diabetes (20-40%). Symptomatic cardiac autonomic neuropathy (CAN) manifests in about 5% of diabetic patients,5 but when present it is associated with the increased mortality attributed to prolonged QT interval predisposing to ventricular arrhythmias, silent ischemia and cardiac arrest.6
CAN early detection and early prevention is essential to prevent such cardiac events. A battery of tests such as heart rate response to valsalva manoeuver, BP response to standing and handgrip, are available but these are cumbersome and not easy to perform in every patient. Therefore, there is a need of simple, non-invasive bed side test to detect early CAN in diabetes. In 1980, for the first time, an association of prolonged QT interval with cardiac autonomic neuropathy was established which opened the possibility of rapid objective method to detect cardiac dysautonomia.
The QT interval in the ECG reflects the total duration of ventricular myocardial depolarization and repolarisation. Increases length of this interval, known as corrected QT (QTc) prolongation, can be a precursor of torsades de pointes, a potentially life threatening ventricular dysrhythmia and ventricular fibrillation 7. Prolongation of QTc increases morbidity and mortality and QTc has been found to be longer in patients with T2DM than in healthy controls. QT prolongation has been shown to predict cardiac death in T2DM. Although there is general agreement that the QT interval is affected by cardiac ischemia, the effect of hyperglycemia on QT measures is controversial. Several studies suggest that assessing the QT interval could be a cost effective way of stratifying such patients according to cardiovascular risk so that aggressive treatment could be directed appropriately to improve outcome8.
Subjects and Method– This cross sectional study was conducted in the Department of Medicine Lady Hardinge Medical College New Delhi. 160 T2DM subjects full filling the selection criteria are enrolled for study from November 2017 to April 2018.
INCLUSION CRITERIA:
- Subjects already diagnosed (less than 5 years since diagnosis) to be having T2DM on diet control/drugs or both.
- New diagnosed (as per ADA 2015 criteria) cases of type 2 diabetic patients on life style modification were included for the study as cases.
- Those with age more than 35 years and both genders were included.
EXCLUSION CRITERIA:
- Type 1 diabetes mellitus.
- Subjects with known case of structural heart disease, unstable angina, arrhythmia, myocardial infarction, cerebrovascular disease, long QT syndrome, electrolyte imbalance, hypo/hyperthyroidism.
- Subjects taking drugs that alter QTC/QTD interval e.g. antiarrhythmic, antihistaminic (amantadine), sparfloxacin, macrolides, antimalarial
- Pregnant female, critical, non -willing
- Subjects having severe derangement in renal function test (serum creatinine >2.0 mg/dl
DATA COLLECTION: Detailed history & examination (including anthropometry and thorough systemic examination) of all the cases was done & subjects was called on another date after 8 hours of overnight fasting for biochemical and pathological tests. Other investigation like 12 lead ECG with rhythm strips of lead 2, urinary tests were also carried out on the day of next visit. Blood pressure measurement (cut off upper limit 139 mmHg SBP, 89mmHg DBP). Arterial blood pressure (BP) was taken with a standard mercury blood pressure instrument (aneroid sphygmomanometer with stethoscope) after 5 minutes of seated rest. Pulse pressure was measured as difference of systolic and diastolic pressures in mmHg. High pulse pressure corresponded to a PP equal or higher than the 75th percentile of the overall PP values for each gender. A value 30-60 mmHg will be taken up as a normal. Approximately 7ml of fasting venous blood sample was extracted with all aseptic precaution for carrying out routine tests like complete blood count (CBC), Liver Function tests (LFT), kidney function tests (KFT), fasting blood glucose (FBG), lipid profile, and HbA1c, Insulin level.
Out of 7ml blood- 3ml collectedin EDTA vaccutainer for CBC and HbA1C, 3ml collectedin plain vacutainer for LFT, KFT, lipid profile. Out of this blood sample 0.5ml serum was stored at -20 degree Celsius for batch analysis for insulin level by HOMA-IR. 1ml kept in fluoride vaccutainer for FBG. Serum was extracted by centrifugation.
Materials required for HbA1c: Hb denaturation reagent, Total Hb reagent, HbA1c R1: Antibody reagent HbA1c R2: Agglutinator reagen. Assay was calibrated by using RandoxHaemoglobinA1c Calibrator series, levels 1-6. With (Lot n. 1600HA),10microlitre of whole blood sample was mixed with 400microlitre of haemoglobin denaturant reagent (1:41 dilution) then incubated for 5 minutes at room temperature prior to testing. Then tests were performed on AU-480 fully automated analyser.
Normal Range: 4-5.6%
Increased risk of Diabetes: 5.7-6.4%
Diabetes Mellitus: ≥6.5%
Estimation of Homeostasis Model of assessment Insulin Resistant (HOMA-IR) by fasting serum insulin
Method: chemiluminescence immunoassay by Beckman Coulter (United States) Access 2 chemiluminiscent Auto-analyser.
Principle: The Access Ultrasensitive insulin assay is a competitive binding receptor assay. For the assay of insulin in serum, no pretreatment is required. Insulin binding protein, mouse anti insulin binding protein, insulin alkaline phosphatase conjugate, and goat anti insulin antibody coupled to paramagnetic particles are added to the reaction vessels. Insulin in the sample competes with the insulin-alkaline phosphatase conjugate for binding sites on a limited amount of insulin binding protein. Resulting materials are washed away. Then, the chemiluminescent substrate lumi-phos 530 was added, complexes bind to the solid phase via mouse anti insulin binding protein. Measurement of serum fasting insulin while unbound to the vessel and light generated by the reaction is measured with a luminometre. The light production is inversely proportional to the concentration of insulin in the sample. The amount of analyte in the sample is determined from a multipoint calibration curve.
HOMA-IR-
Calculation formula: = Fasting insulin (U/l) fasting glucose (mg/dl)
405
Category HOMA-IR Score89
Normal insulin resistance <3
Moderate insulin resistance 3-5
Severe insulin resistance >5
Urinary analyses: Urine sample was obtained in appropriate container for urine routine microscopy to rule out albuminuria. Urine for micro-albuminuria and albumin-creatinine ratio (ACR) was carried out where-ever required.
12 LEAD ELECTROCARDIOGRAPHY: 12 lead Electrocardiography including Lead 2 rhythm strip was done in all subjects. RR & QT Interval were measured manually with a ruler on the resting ECG tracing. Three consecutive beats were considered on the 12 lead ECG to measure QTc and QTd. QT interval measure from beginning of the QRS complex to end of the down slope of the T wave. QT interval vary according to the heart rate, it was corrected using Bazett’s formula– QTc = QT/RR1/2 to give QTc interval, and to found (maximum, minimum, and mean). A QTc value >440msec was considered as prolonged in males and >460msec was considered as prolonged in females90.QTd is defined as the difference between the maximum and minimum QT interval on the 12 lead ECG. There is still some confusion about the upper limit of normal QTd. QTd> 80 ms (0.08s) is usually considered as abnormally prolonged91.
Statistical analysis: All statistical data were compiled, tabulated using MS EXCEL spreadsheet and analyzed using Statistical Package for Social Sciences (SPSS) version 21.0.
Parametric or continuous variables were analyzed for central tendencies using mean, median and mode Significance of difference for parametric variables were determined by unpaired t-student test and the Mann-Whitney test used for nonparametric data. Qualitative variables were correlated using Chi-Square test /Fisher’s exact test. Measures of correlation using Pearson’s and Spearman’s correlation coefficient was applied to determine the association between variables value less than 0.05 was considered as significance.
Results: Total 160 diabetics including as a cases attending Medicine OPD, inpatients, fulfilling the inclusion criteria were studied. descriptive data as table -1 average age of subjects was 52.01±8.39 year with mean duration of disease 2.47± 1.45 years, mean BMI was 26.13±3.4, mean WC was 93.86±7.24 cm, mean PP was 50.44±12.03 mmHg, mean FBG was 159.69±40.93 mg/dl, HbA1c was 8.29%±1.84%, average HOMA-IR was 4.26±1.76, mean QTc interval was 422.52±41.07 and mean QTd interval was 58.19±32.43. 55% study subjects were male, 72.51% subjects were in age group of 41-60years, 70% subjects were in obese category. In study population prevalence of wide PP was 31.30%, QTc prolongation was 33.75% and QTd was 37.50%. Abnormal HOMA-IR was in 70.63% study subjects. The prevalence of microvascular complications like retinopathy, nephropathy and neuropathy were 30.63%, 25.63% and 18.13% respectively.
Table 1: Descriptive characteristic of study population
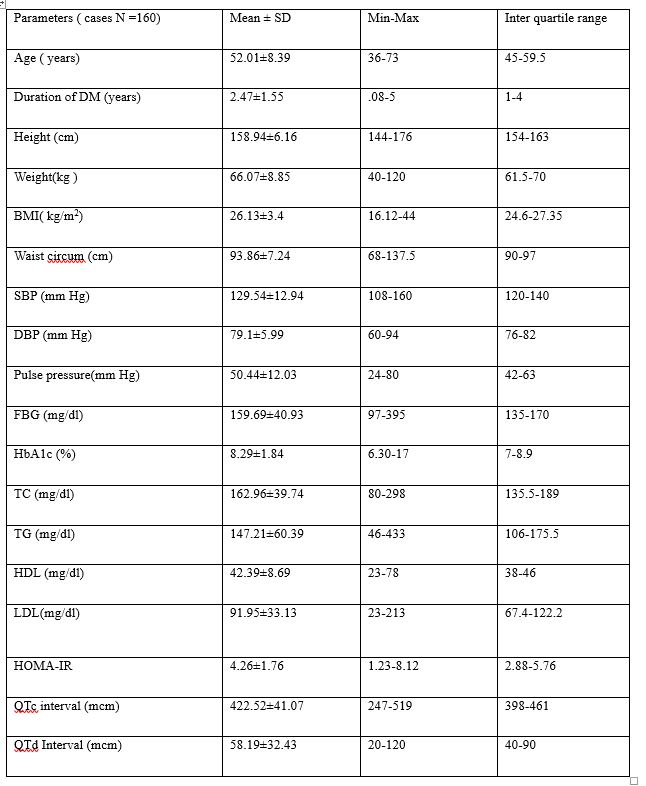
We found significant difference when pulse pressure was compared with respect to various age group (56.12 ± 7.51 vs 50.14 ± 8.13, p<0.0001), mean duration of illness (3.13±1.44 vs 2.16±1.51, p=0.0004), WC (26.17±2.82 vs 26.11±3.64, p=0.001), FBG (166.54±45.48 vs 156.58±38.5, p=0.04), HbA1c (9.09±2.2 vs 7.08±1.02, p=0.0004), TG (157.46±46.07 vs 142.54±65.54, p=0.014), HOMA-IR (4.89±1.58 vs 3.98±1.77, p=0.001), QTc interval (446.48±29.6 vs 411.64±41.03, p<0.0001), QTd interval (76±31.49 vs 50.09±29.6, p<0.0001) as showed in table 2. There was no any significant difference was observed when PP was compared with respect to different mean BMI, TC, HDL and LDL groups. There was further Significant difference was documented when QTc interval was compared with respect to mean duration of illness (3±1.51 vs 2.2±1.5, p=0.002), PP (56.48±13.24 vs 47.36±10.12, p<0.0001), HbA1c (9.03%±2.18% vs 7.92%±1.51%, p=0.0001), TG (157.37±45.48 vs 142.03±66.31, p=0.007), HOMA-IR (4.97±1.61 vs 3.9±1.73, p=0.0002) and QTd interval (80.93±26.3 vs 46.6±29.01, p<0.0001). This result also revealed that no any significant difference was there when QTc interval was compared with reaspect to various mean age, WC, BMI, FBG, TC, HDL and LDL groups (table 2). Table 2 also showing that significant difference was observed when QTd interval compared with respect to various age group (55.42±7.33 vs 49.96±8.36, p=0.0001), mean duration of type 2 diabetes (3.03±1.46 vs 2.13±1.51, p=0.001), PP (57.13±13.32 vs 46.42±9.12, p<0.0001), HbA1c (8.91%±2.13% vs 7.93%±1.53%, p=0.001), TG (159.9±49.16 vs 139.59±65.27, p=0.002), HOMA-IR (4.93±1.74 vs 3.86±1.65, p=0.0003) and QTc interval (445.92±37.78 vs 408.49±36.43, p<0.0001). This results also demonstrated that no any significant difference was noticed when QTd interval compared with respect to different mean BMI, mean WC, mean FBG.
Table 2:- clinical & laboratory parameters association with Pulse pressure, QTc & QTd interval
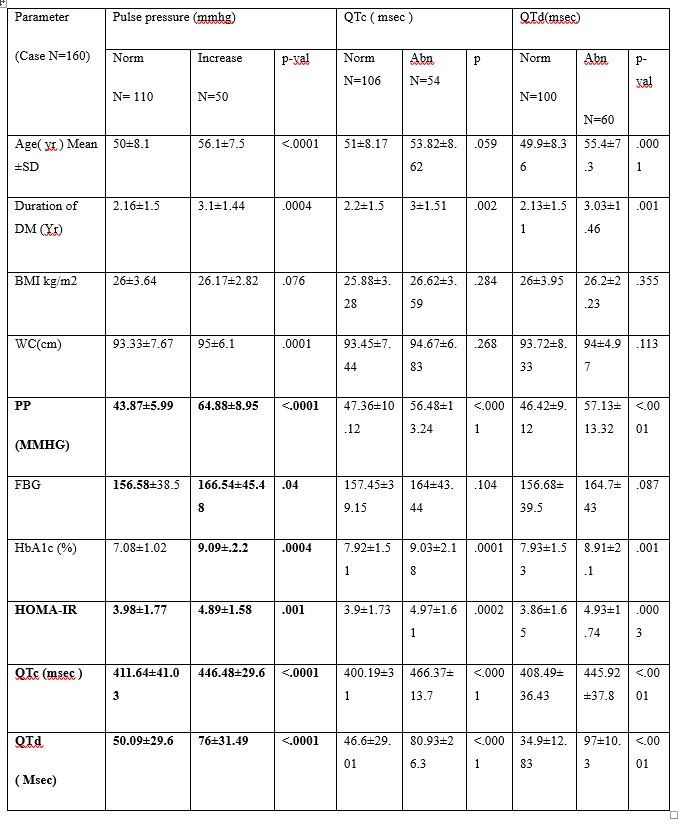
Documented significant difference when retinopathy was compared with respect to various mean pulse pressure group (48.65±10.47 vs 54.49±14.29, p=0.02), mean QTc interval group (412.58±39.77 vs 445.06±34.86, p<0.0001) and mean QTd interval group (50.36±29.45 vs 75.92±32.14, p<0.0001). There was also showing significant difference was documented when nephropathy was compared with respect to different mean QTc interval group (418.44±41.09 vs 434.37±39.09, p=0.03) and mean QTd interval group (54.45±30.83 vs 69.02±34.84, p=0.014). No significant difference was found when nephropathy was compared with respect to different mean PP groups. We observed significant difference when neuropathy was compared with respect to various mean QTc interval group (419.13±40.61 vs 437.86±40.25, p=0.025) and mean QTd interval group (55.04±31.09 vs 72.41±35.02, p=0.010). No significant difference was found when neuropathy was compared with respect to different mean PP groups as mentioned in table 3.
Table: 3 PP & Electrocardiographic factors association with various complication of DM
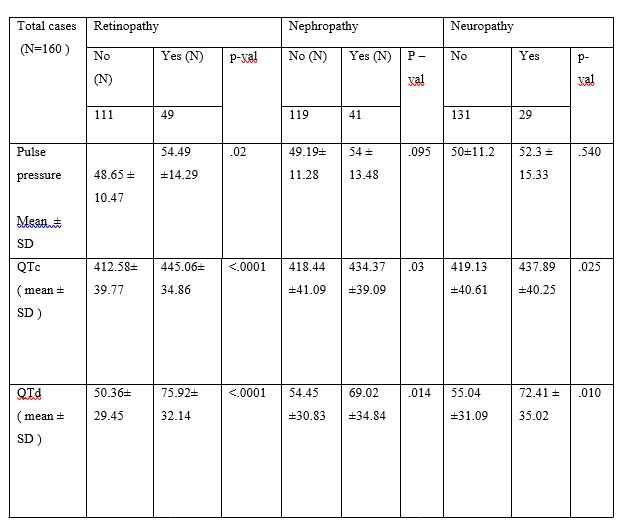
In our study as tabulated in table 4 QTc interval was significantly associated with HbA1c (r=0.291, p=0.0002) (table 4 figure 1) and HOMA-IR(r=0.273, p=0.0005) (table4 figure 2). Similarly age, duration of type 2 diabetes, BMI, and TG also showed positive correlation to QTc interval. QTc interval was not significantly associated with FBG (r=0.061, p=0.4435). Similarly TC, HDL, LDL and WC also showed not significant association with QTc interval. QTd interval was significantly associated with HbA1c (r=0.232, p=0.0031) (table4 figure 3) and HOMA-IR (r=0.246, p=0.0017) (table 4 figure 4). Similarly age, duration of illness, and TG also documented significant correlation with QTd interval. QTd interval was not significantly associated with FBG (r=0.151, p=0.0561). Similarly BMI, TC, HDL, LDL and WC also showed no any significant correlation with QTd interval.
PP was significantly associated with HbA1c (r=0.215, p=0.0062) (table 4 figure 5) and HOMA-IR (r=0.251, p=0.0013) (table 4 figure 6). Similarly significant association also observed between age, duration of illness, BMI, WC, TG and PP. PP was not significantly associated with FBG (r=0.074, p=0.3518). Similarly PP was not significantly correlated with TC, HDL and LDL.
Table:-4 Correlation of Electrocardiographic changes (QTc & QTd Interval) and Pulse Pressure with various Biochemical parameters



Discussion: The main finding of this study was that wide Pulse Pressure quite prevalent (31.30%) in type 2 diabetes mellitus. Similarly QTc and QTd abnormalities with prolonged QTc and QTd duration (mean= 422.52±41.07 and 58.19±32.43 respectively) were recorded in a very high frequency (33.75% and 37.50% respectively) in T2DM.
Veglio et al 9 found the prevalence of QTc and QTd interval prolongation were 26% and 33% respectively in type 2 diabetics. Abdul et al10 revealed 31.6% QTc and 42.1% QTd prolongation in T2DM. VM Ninkovic et al11 reported prolongation of QTc and QTd in 44.1% and 3.6%. In this study abnormal HOMA-IR was 70.63% (N=113) with 30.63% (N=49), 25.63% (N=41) and 18.13% (N=29) prevalence of retinopathy, nephropathy and neuropathy .while Zhaolan Liu et al12found the prevalence of retinopathy, nephropathy and neuropathy was 6.1%, 10.7% and 17.8% respectively.
In this study PP was significantly associated with HbA1c (r=0.215, p=0.0062). However Jun et al13 did not find correlation between PP and HbA1c (p=0.64,r=-0.034). Similarly HOMA-IR in our study had a positive correlation (r=0.251, p=0.0013) with PP. Jun et al13had a different observation from our study (p=0.5,r=0.068). PP was also found significantly associated with age (r=0.293, p=0.0002).Similar results was observed by Schram MT et al14 (p<0.001) and Jun et al (p<0.01,r=0.473). In this study BMI had a significant association (r=0.287, p=0.0002) with PP. This was not in agreement with the Jun et al in which bivariate correlation between brachial PP and BMI was not significant (p=0.56,r=0.04). WC was also found positive correlation with PP in our study (r=0.423, p<0.0001). No similar study was found in literature review. In addition to these duration of illness (r=0.232, p=0.0031) and TG (r=0.256, p=0.0011) was also observed significant relationship with PP. This implies that increased arterial stiffness observed with long term poor glycemic control, insulin resistance, increasing age and duration of illness, obesity and dyslipidemia.
In this study QTc interval was significantly associated with HbA1c (r=0.291, p=0.0002). Timar et al15 (p=0.041) and VM Ninkovic et al11 (p=0.001) were support our result. Here we observed significant relationship between QTc interval and HOMA-IR (r=0.27 ,p=0.0005). Timar et al15 reported that QTc interval is strongly positive and statistically significant correlated with HOMA-IR values (Pearson r=0.62; p< 0.001). QTc interval was also found significantly associated with age (r=0.217, p=0.0059). Similar as Timar et al15 (p=0.048) and V M .Ninkovic et al11 (p=0.017). Similarly, in this study BMI was significantly associated (r=0.156, p=0.0495) with QTc interval. This result was like VM Ninkovic et al11 (p=0.005). In addition to these results in our study QTc interval also significantly associated with duration of type 2 diabetes (r=0.269, p=0.0006) and TG (r=0.304, p=0.0001). This result has documented that the cardiac autonomic neuropathy manifested as increased QTcinterval could be linked to long term hyperglycemia, insulin resistance, increasing age and duration, obesity, dyslipidemia.
In our study QTd interval was significantly associated with HbA1c (r=0.232, p=0.0031). Here we found HOMA-IR was significantly associated with QTd (r=0.246, p=0.0017). No such kind study reported in literature. QTd interval had significantly associated with age (r=0.27, p=0.0006) in this study. This result was similar as VM Ninkovic et al11 (p=0.023). In this study duration of illness (r=0.296, p=0.0001) and TG (r=0.257, p=0.001) were found significantly correlated with QTd . These results had shown that increased ventricular excitability was associated with poor long term glycemic control, insulin resistance, increasing age, increasing duration of illness and dyslipidemia.
In this study out of our aims FBG was not significantly associated with PP (r=0.074, p=0.3518) and QTc (r=0.061, p=0.4435). Which was conflicting as VM Ninkovic et al11 (p<0.001) . QTd had no significant association with FBG here (r=0.151, p=0.0561). similar result by VM Ninkovic et al11 (p=0.305). This implies that acute or short term glycemic status may not have any significant effect on myocardial excitability and arterial stiffness.
This study also revealed significant association of PP with retinopathy (X2=12.850, p=0.0003). Our result has similarity with previous researchers (IshiharM et al16 (60.5 vs 56.4, p<0.001) and Mbenza B et al17 (56.9±18.3 vs 49.5±15.2, p<0.001). In our study QTc interval was positive correlation with retinopathy (X2=20.434, p<0.0001) and nephropathy (X2=3.909, p=0.048). Which were similar to Aboelnagaet al 18. This confirmed the cardiac autonomic neuropathy linked with microvascular complications like retinopathy and nephropathy.
This study also revealed significant association of QTd interval with microvascular complications like retinopathy (X2=20.006, p<0.0001), nephropathy (X2=4.427, p=0.035) and neuropathy (X2=4.720, p=0.030). These results indicate that ventricular excitability correlate significantly with microvascular complication.
The EURODIAB investigators point out that prolongation of the QT interval corrected for heart rate (QTc) is a strong risk factor “for all-cause and cardiovascular mortality in apparently healthy people, as well as in people with various conditions, including diabetes. Prolonged QTc interval may be a marker of subclinical undetected atherosclerotic process, and QTd has been shown to predict mortality in type 2 diabetic patients. Prevalence of prolong QTc and QTd increase in diabetics than control non diabetics19.
It was previously suggested that hyperglycaemia may lead to QT prolongation through several mechanisms including stimulation of protein kinase C leading to reduced synthesis and release of endothelial derived nitric oxide. This consequently lead to decrease in activity of Na+ K+ ATPase, an enzyme responsible for the maintenance of basal membrane potential of myocytes, and cells of the conduction system (by maintaining a high concentration of intracellular K and high concentration of extracellular Na by mechanism of active transport)20. Reduced nitric oxide bioavailability during hyperglycaemia is probably responsible for decreased activity of Ca2+ ATPase as well, an enzyme in the membrane of myocytes that through active transport mechanism maintains a low concentration of Ca2+ ions in the cell, resulting in an increase in the influx of Ca2+ ions during phase 2 of the action potential and extension of QT interval 21.
The mechanism of QTc prolongation with insulin resistance: High insulin levels are associated with increases in sympathetic nerve activity, which in turn enhances myocardial cell membrane refractoriness and thus prolongs the QTc interval22,23. Insulin can also cause hypokalemia, which often results in a prolongation of QTc interval. The mechanism has association PP, QTc and QTd interval with microvascular complication in T2DM still not known .
The mechanism of wide PP in aging could be as a result of changes in intima media of the arterial wall as a consequence of fractures of the elastic lamina, loss of muscle attachments, increase in collagen fibers, local inflammation, infiltration of vascular smooth muscle cells and macrophages, fibrosis, deposition of mucoid material, focal media smooth muscle cell necrosis, and calcification. The intima-medial thickness triples between the ages 20 and 90. A major component of this compositional change with aging is a consequence of elastin fracture, with elastin being progressively replaced by collagen24. The mechanism of wide PP in obesity may be explained bythe hypothesis that people with a high BMI and high WC also have high PP because obesity is associated with arterial stiffness.
The possible mechanism of prolongation of QTc interval in advance age may be due to arterial stiffness increasing with age. This could result in wide pulse pressure and hypertension in elderly age group. Hypertension is frequently associated with left ventricular hypertrophy and also associated with sympathovagal imbalance characterized by vagal withdrawal and relative sympathetic dominance25,. Structural changes in the hypertrophied myocardium, altered ion channels operating during the early repolarization phase, and fibrotic changes in the myocardium due to left ventricular hypertrophy may lead to the prolongation of QTc interval.
The possible mechanism of QTc prolongation in obesity has been associated with autonomic nervous system dysfunction. This may result in prolongation of the QTc interval (25).
El-Gamal et al26 suggested that an elevated cardiac output in obesity, due to an increased stroke volume and overall greater body mass, may contribute to alter autonomic nervous system balance. It is possible that some unknown dysfunction in autonomic nervous system balance could have influenced the findings of increased QTcinterval in this study.Another possible mechanism contributing to prolongation of the QTcinterval in obesity may be electrolyte abnormalities.
Strengths of this study:
This was the first study which studied multiple risk factors like Pulse Pressure (PP), QTc interval and QTd interval and its association with insulin resistance (HOMA-IR) , FBG and HbA1c in type 2 diabetes mellitus.
This study established significant association between QTd and microvascular complications like retinopathy, nephropathy and neuropathy. These could interm indicating degree of myocardial excitability in subjects having microvascular complication.
Limitations of this study:
- This was a cross sectional study not a representative of the entire population, swe could not determined the cause and effect relationship of PP, QTc interval and QTd with HbA1c, FBG and HOMA-IR.
- We did not correlate the parameters with any undiagnosed cardiac structural abnormalities, as we have not carried out other investigation like Holter, heart rate viability (HRV) & 2D-ECHO.
Conclusion: This study concluded that type 2 diabetics had a high prevalence of wide Pulse Pressure with prolonged QTc and QTd interval, thereby indicating increased arterial wall stiffness and possible presence of CAN.
The PP, QTc and QTd were significantly associated with long term glycemic. These parameters were notably associated with increasing degree of insulin resistance, thereby indicating possible link of insulin resistance with arterial stiffness and CAN. Prolonged QTd was the key determinant which had correlation with complications (retinopathy, nephropathy and neuropathy), patients with above complications should be examined for CAN on prime concern. Simple bed side test pulse pressure and simple ECG ( QTc, QTd ) would be easiest marker of CAN and Determinants of further Complications of DM.
Conflict of Interest : Nil
Support : Nil
Acknowledgements: Nil
References:
1.World Health Organization, Global Report on Diabetes. Geneva, 2016. Accessed 30 August 2016.
- Chisholm DJ, Campbell LV, Kraegen EW. Pathogenesis of the insulin resistance syndrome (syndrome X). ClinExpPharmacol Physiol. 1997;24:782–784
3.Gerick JE. The genetic basis of type 2 diabetes mellitus: impaired insulin secretion versus impaired insulin sensitivity. Endocr Rev. 1998;19:491–503.
- Gray RS, Fabsitz RR, Cowan LD, Lee ET, Howard BV, Savage PJ. Risk factor clustering in the insulin resistance syndrome: the Strong Heart Study. Am J Epidemiol. 1998;148:869–878.
- Dejgaard A Pathophysiology and Treatment of Diabetic Neuropathy. Diabetic Medicine 1998;15:97-112.
6.Rathmann W, Ziegler D, Jahnke M, Hasstert B, Gries FA. Mortality in diabetic patients with cardiovascular autonomic neuropatrhy. Diabetic Med 1993;10: 820-24.
- Ewing DJ, Martyn CN, Young RJ, Clarke BF. The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care 1985;8:491-98.
- Rajeev Kumar, Miles Fisher, Peter W Macfarlane Diabetes and the QT Interval: Time for Debate, British Journal of Diabetes and Vascular Disease. 4(3):146-150, 2004.
- Veglio M, Bruno G, Borra M et al. Prevalence of increased QT interval duration and dispersion in type-2 diabetic patients and its relationship with coronary heart disease: a population-based cohort. J Inter Med; 2002;25,317-24.
- Faris Abdul Kareem Khazal. Qtc and Qtd Intervals in Patients with Type 2 Diabetes Complicated by Peripheral Sensory Neuropathy. THE IRAQI POSTGRADUATE MEDICAL JOURNAL, 2008, VOL.7,NO.3, p.249-253
- Vladan M. Ninkovic, Srdjan M. Ninkovic, VanjaMiloradovic, DejanStanojevic, MarijanaBabic, Vojislav Giga, Milan Dobric, Michael I. Trenell, NebojsaLalic, Petar M. Seferovic, Djordje G. Jakovljevic. Prevalence and risk factors for prolonged QT interval and QT dispersion in patients with type 2 diabetes. ActaDiabetologica, October 2016, Volume 53, Issue 5, pp 737–744.
- Zhaolan Liu1, Chaowei Fu, Weibing Wang and Biao Xu. Prevalence of chronic complications of type 2 diabetes mellitus in outpatients – a cross-sectional hospital based survey in urban China. Liu et al. Health and Quality of Life Outcomes 2010, 8:62.
- Jun Liang , Na Zhou , FeiTeng, CaiyanZou, Ying Xue, Manqing Yang, Huaidong Song, Lu Qi. Hemoglobin A1c Levels and Aortic Arterial Stiffness: The Cardiometabolic Risk in Chinese (CRC) Study. August 3, 2012, DOI: 10.1371/journal.pone.0038485.
- Schram MT, Kostense PJ, Van Dijk RA, Dekker JM, Nijpels G, Bouter LM, Heine RJ, Stehouwer CD. Diabetes, pulse pressure and cardiovascular mortality: the Hoorn Study. Journal of Hypertension 2002 Sep;20(9):1743-51.
- Romulus Timar, SimonaPopescu, MihaelaSimu, Laura Diaconu, BogdanTimar. QTc INTERVAL AND INSULIN RESISTANCE IN TYPE 2 DIABETES MELLITUS. European Scientific Journal, April 2013 edition vol.9, No.12, ISSN: 1857 – 7881 (Print) e – ISSN 1857- 7431
- Ishihara M, Yukimura Y, Aizawa T, Yamada T, Ohto K, Yoshizawa K. High blood pressure as risk factor in diabetic retinopathy development in NIDDM patients. Diabetes Care. 1987 Jan-Feb;10(1):20-5.
- Longo- Mbenza B, NkondiMbadiANsungu J, MbunguFuele S. Higher pulse pressure, systolic arterial hypertension, duration of diabetes and family history of diabetes in Central Africans. Int J Diabetes & Metabolism (2008) 16: 17-23.
- Mohamed M. Aboelnaga, Maha M elshafei, EmanElsayed. Prevalence and Risk Factors of Prolonged QTc Interval among Egyptian Type 2 Diabetes Patients. JMSCR, July 2016, VOL.4, Issue7, pp:11326-11333.
- Gupta S, Sussman I, McArthur CS, Tomheim K, Cohen RA, Ruderman NB (1992) Endothelium-dependent Inhibition of Na+-K+ ATPase activity in rabbit aorta by hyperglicemia. Possible role of endothelium-derived nitric oxide. J Clin Invest. 90:727–732
- Davis FB, Davis PJ, Nat G et al (1985) The effect of in vivo glucose administration on human erythrocyte Ca2+ -ATPase activity and on enzyme responsiveness in vitro to thyroid hormone and calmodulin. Diabetes 34(7):639–646
21. Dekker JM, Feskens EJM, Schouten EG, et al. QTc duration is associated with levels of insulin and glucose tolerance. The Zutphen elderly study. Diabetes 45 (1996): 376-380.
- Ferrannini E, Galvan AQ, Gastaldelli A, et al. Insulin: New roles for an ancient hormone. Eur J Clin Invest 29 (1999): 842-852.
- M. F. O’Rourke and J. Hashimoto, “Mechanical factors in arterial aging: a clinical perspective,” Journal of the American College of Cardiology, vol. 50, no. 1, pp. 1–13, 2007.
- Passino C, Magagna A, Conforti F, et al. Ventricular repolarization is prolonged in nondipper hypertensive patients: role of left ventricular hypertrophy and autonomic dysfunction. Journal of Hypertension. 2003;21(2):445–451
- Moss AJ. Prolonged QT interval syndrome. JAMA 1986; 256: 2985±2987
- El-Gamal A, Gallagher D, Nawras A, Gandhi P, Gomez J, Allison DB, Steinberg JS, Shumacher D, Blank R, Heymsfield SB. Effects of obesity on QT, RR, and QTc intervals. Am J Cardiol 1995; 75: 956±959.