https://meditropics.com/monoclonal-antibody-in-malignancy/
Shivshankara Swamy Mathighatta Shivarudraiah Consultant, Department of Medical Oncology,Rajiv Gandhi Cncer Institute & Research Center, New Delhi
Introduction
Monoclonal antibodies (mAbs) are a relatively new class of biologics that have revolutionized the treatment landscape and outcome of various malignancies(1). Monoclonal antibodies are sticky proteins made by the single clone of plasma B cells that have high specificity and affinity for the antigen they were generated against. Once attached, they recruit other parts of the immune system to destroy the target containing the antigen. Sir Gustav Nossal showed that a single B cell clone produces one specific antibody. Köhler and Milstein developed hybridoma cells by fusing mouse spleen and myeloma cells to produce a single antibody clone in large volumes with a pre-selected specificity, which became known as mAbs(1,2).
The initial murine origin mAbs are extensively used in basic research and diagnostic tests. However, their therapeutic usage quickly became problematic as they were highly immunogenic and non-sustainable for long-term therapy due to the development of human anti-murine antibodies(3). Immunogenicity led to increased incidence of infusion reaction and reduced efficacy. Engineering innovations paved the way for humanized antibodies and “fully-human” antibodies in order to eliminate these limitations. Cancer cells are an end result of multiple aberrations in the cell genome, as a result they express some abnormal proteins or overexpress certain markers. Therapeutic mAbs can be designed to specifically target a protein that is deregulated during tumorigenesis and arrest cancer cell growth and survival. Thereby, mAbs have become an important modality in the treatment of cancer.
Monoclonal Antibody Nomenclature
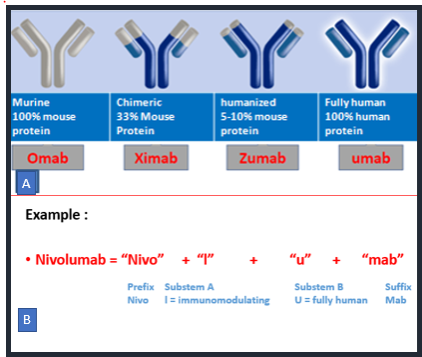
Over the years, the World Health Organization (WHO) has established guidelines for implementing a consistent nomenclature system for mAbs resulting in the International Nonproprietary Name (INN). The INN nomenclature of mAbs was assembled from a randomly chosen prefix followed by a substem A, substem B and suffix i.e universal stem “mab”(3). Substem A represents the specific target against which antibodies are developed such l(i)- for immunomodulating or -t(u)- for tumor and substem B indicate whether they are derived from mouse (-omab), chimeric (-ximab), humanized (-zumab) or fully human (-umab) as shown in figure 1. Substem B has been omitted from nomenclature after mid 2017 (ex: cemiplimab). In 2021 INN nomenclature scheme has introduced a new suffix instead of mab which divides the mAbs into four groups, three groups for monospecific immunoglobulins and one for bi- and multi-specific immunoglobulins. The four groups in the INN 2021 nomenclatures are group 1 with suffix -tug for unmodified immunoglobulins, group 2 -bart for artificial antibody such as full length immunoglobulins with engineered constant domains, group 3 -ment for fragment of any kind, derived from an immunoglobulin variable domain and group 4 -mig for Bi- and multi-specific immunoglobulin(4).
Figure 1: International Nonproprietary Name (INN) of monoclonal antibodies.
Monoclonal antibodies structure and mechanism of action
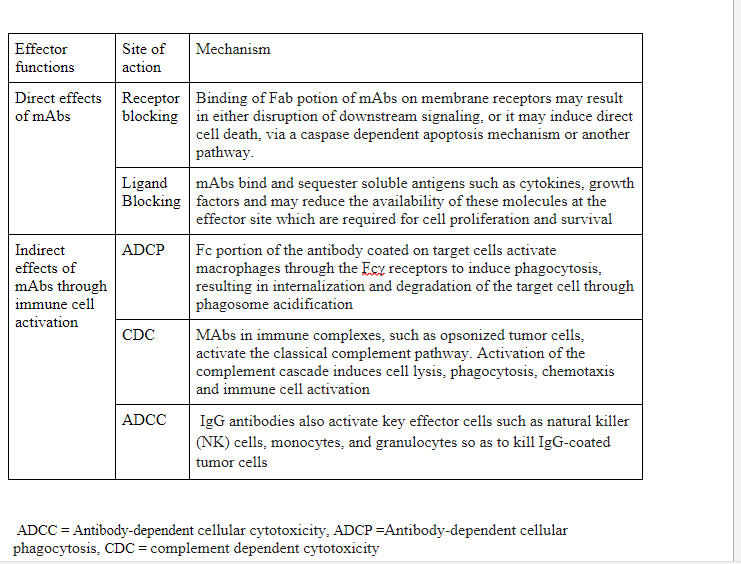
Immunoglobulin G (IgG) class antibodies are the most commonly used mAbs. IgG has four subclasses from IgG1 to IgG4. Monoclonal antibodies are usually made up of two antigen-binding fragments (Fabs) and one fragment crystallizable (Fc) region, which interacts with Fc receptors (FcRs) on various immune cells(1). With the Fc region, antibodies bind to several types of receptors, such as Fc gamma receptors (FcγR), a complement receptor, and a neonatal Fc receptor. Fc engineering is one of the newer innovations to maximize the value or overcome the drawbacks of monoclonal antibodies for therapeutic use. Functionally, IgG1 and IgG3 are potent inducers of Fc-mediated effector mechanisms such as antibody-dependent cellular cytotoxicity (ADCC), antibody- dependent cellular phagocytosis (ADCP), and complement dependent cytotoxicity (CDC)(1).
Monoclonal antibodies structure may vary from small fragments to intact immunoglobulins, modified, or unmodified, all of which contain an antigen binding domain (Fabs), and accordingly they are labeled as Fab fragments, naked mAbs, conjugated mAbs and bispecific antibodies. Fab fragments are fragments of antibodies instead of intact Ig that may improve efficiency of penetration into tissues or tumor masses. Fab fragments lack the Fc component of the antibody and thus are not capable of interacting with Fc receptors or activating complement. Naked mAbs are antibodies that work by themselves and do not carry any additional payloads. Whereas conjugated mAbs are combined with a chemotherapy drug or a radioactive particle.
Depending on the location, antigens are classified into two groups, soluble antigen and membrane-bound antigen. Soluble antigens may be cytokines (TNFα, IL-6) or growth factors (VEGF). Membrane-bound antigen targeting antibodies are the most commonly used mAbs in therapeutic use, which include tumor surface markers (HER2, CD20, CD19), receptors (EGFR, VEGFR, HER2 receptor). Receptor-targeting antibodies perform one of two roles, direct effect through interaction through membrane bound receptor and indirect action through immune system activation by linking pathogenic and effector cells as described in Table 1.
Therapeutic monoclonal antibodies used in oncology
The mainstay of cancer treatment has been chemotherapy for longtime, however they affect all dividing cells including normal cells like blood cells, mucosal epithelium causing many adverse effects with narrow therapeutic window. In order to overcome this bottleneck, new classes of drugs have risen which specifically targets cancer cells. Large number of trials have been done and are ongoing to develop personalized medicine which specifically targets cancer cells thereby improving efficacy and reducing toxicity. Monoclonal antibodies are a prime example of personalized medicine as result of advances in the field of molecular biology and immunology. As an example, cancer cells can be evaluated for the presence of certain markers (ex: Her2neu expression in breast cancer cells), which can, in turn, be targeted by mAbs (i.e. Trastuzumab) to provide a “tailored” therapy. Number of therapeutic mAbs approved has substantially grown in the last decade exceeding 100 mAbs with nearly 50 mAbs in Oncology alone.
HER2
HER2 is part of the EGFR family of receptors commonly implicated in cancers such as breast cancer, gastric cancer, colorectal cancer and lung cancer. Multiple mAbs have been approved for treatment of Her2 receptor overexpressed cancers like Trastuzumab, Pertuzumab. Binding of mAbs to HER2 receptor interferes with receptor dimerization, which disrupts the intracellular signaling cascade, inhibiting cell growth. Her2 positive breast cancer are the most aggressive subtypes of breast cancer with poor outcomes and survival less than 2 years. Usage of these monoclonal antibodies have dramatically improved outcomes with median survival reaching beyond 5 years for metastatic Her 2 positive breast cancer(5).
CD20
More than ninety percent of B-cell lymphomas express the cluster differentiation 20 (CD20) antigen. Rituximab, a chimeric monoclonal antibody that targets CD 20, is approved for use in CD 20 positive chronic lymphocytic leukemia and various B cell non-Hodgkin’s lymphoma. Rituximab by binding to CD20 on B-cells surface has been shown to induce direct cell death, and also activates antibody-dependent cell-mediated cytotoxicity on flagged B cells.
Immune checkpoint inhibitors (ICI)
Immune surveillance is the interplay between the immune system and tumors wherein the immune system recognizes and destroys mutant cells which have the potential to develop into tumors, however some cancer cells acquire the ability to evade the immune system and grow unimpeded. Cancer cells evade the immune system by different immune checkpoint pathways. Examples of immune checkpoints are programmed cell death 1 (PD-1) expressed on T cells, B cells, and NK cells. It binds to the programmed cell death ligand 1 (PD-L1) expressed on the surface of multiple tissue types, including many tumor cells. The PD-1:PD-L1 interaction results in peripheral T effector cell exhaustion, and evasion from immune surveillance(6). Similarly CTLA-4 acts as a negative regulator of T cell activation. Monoclonal antibodies that target immune checkpoints relieve the physiological brake on immune activation.
The paramount success in cancer treatment in the last decade has been the introduction of the CTLA-4, PD1 and PDL1 inhibitors. Ipilimumab, the first antibody blocking an immune checkpoint (CTLA4) approved for treatment of melanoma, soon several PD 1 inhibitors (Nivolumab, Pembrolizumab) and PDL1 inhibitors (Atezolizumab, Durvalumumab) have flooded in for the treatment of various cancers like lung cancer, melanoma, Hodgkin’s lymphoma, Gastric cancer, cervical cancer, Hepato-cellular carcinoma, renal cell carcinoma and many more(6). Several biomarkers are being used to predict efficacy of ICIs such as PD L1 expression, tumor mutation burden, tumor infiltrating lymphocytes and MSI (microsatellite instability) status. One of the impressive results of ICIs has been long sustained remission, raising substantial hope for cure for some patients.
Bispecific Antibodies
Bispecific monoclonal antibodies (BsAb) are made up of parts of two immunoglobulin chains of differing specificity that have been combined to form a single antibody molecule. This brings two cells bearing two different antigen like cancer cell and immune cell into close physical proximity. The most widely used BsAb is a bispecific T cell engager (BiTE), with one part binding CD3 surface marker on T cells and the other recognizing target proteins on tumor cells, thereby bringing the T cells to recognise and kill the tumor cells. One first-in-class BiTE is blinatumomab, which targets both CD19 and CD3, is used for the treatment of patients with relapsed and/or refractory or minimal residual disease positive B cell acute lymphoblastic leukemia.
Conjugated monoclonal antibodies
Conjugated monoclonal antibodies are mAbs attached to a drug, radioactive substance, or toxin. The mAb is used as a homing device to take one of these substances directly to the cancer cells thereby reducing systemic exposure(7). This decreases the damage to normal cells in other parts of the body. Brentuximab Vedotin is an antibody drug conjugate (ADC) made up of mAb that targets CD30, loaded with antitubulin agent monomethyl auristatin E via a cleavable linker. Brentuximan Vedotin after binding to CD 30 positive cells, will be internalized and inside the cell the cytotoxic drug monomethyl auristatin E will be released. As a result, cells expressing CD 30 will be selectively killed. Brentuximab vedotin has been approved for CD 30 positive cancers such as Hodgkin’s lymphoma, Anaplastic large cell lymphoma(7). Trastuzumab deruxitican is another ADC approved for treatment of Her2 positive metastatic breast cancer.
Table 1: Effector functions of therapeutic monoclonal antibodies (mAbs)
Limitations
Despite advancements and widespread use, mAbs have their own drawbacks and limitations. Apart from infusion reactions, mAbs also have off site on target side effects such as depleting normal cells expressing target antigens. The efficacy of mABs is limited by poor penetration and heterogeneous uptake in tumor mass due to its bigger size. Cancer cells may adapt by downregulating target antigens and thereby affecting therapeutic efficacy of mAbs. Lastly the cost has been a major limiting factor for wider application of mAbs particularly in resource limited countries like India.
Conclusions
Growing knowledge in the field of tumor immunology and molecular oncology have led to widespread use of therapeutic mAbs. The mAbs are able to disrupt functions of the deregulated proteins on cancer cells, modulate immune response, and deliver cytotoxic payload directly to cancer cell vicinity. Monoclonal antibodies have revolutionized the field of oncology in more than one way, it has changed the way we understand cancers, treatment landscape, adverse effect profile and treatment outcomes.
REFERENCES
- Zahavi D, Weiner L. Monoclonal Antibodies in Cancer Therapy. Antibodies (Basel). 2020 Jul 20;9(3):34.
- Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–7.
- Mayrhofer P, Kunert R. Nomenclature of humanized mAbs: Early concepts, current challenges and future perspectives. Hum Antibodies. 27(1):37–51.
- Balocco R, Koch SDSG, Thorpe R, Weisser K, Malan S. New INN nomenclature for monoclonal antibodies. The Lancet. 2022 Jan 1;399(10319):24.
- Swain SM, Miles D, Kim S-B, Im Y-H, Im S-A, Semiglazov V, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020 Apr;21(4):519–30.
- Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun. 2020 Jul 30;11(1):3801.
- Drago JZ, Modi S, Chandarlapaty S. Unlocking the potential of antibody–drug conjugates for cancer therapy. Nat Rev Clin Oncol. 2021 Jun;18(6):327–44