https://meditropics.com/covid-19-immunology-therapeutics/ Mohit Chowdhury, Sanjana Pant, Niranjan Mahishi, Manish Soneja, Naveet Wig Department of Medicine, AIIMS, New Delhi
The World Health Organization (WHO) declared COVID-19 as a pandemic on March 11, 2020, after it was declared a public health emergency on January 30, 2020. The disease caused by SARS-CoV-2 is known as COVID-19. It has myriad clinical presentations ranging from asymptomatic infection to full blown severe acute respiratory distress syndrome (ARDS). The pathophysiology of severe COVID-19 is divided into two phases: viral and inflammatory. The severity of disease is attributed to immune dysregulation, cytokine release syndrome, inflammasome activation, and interferonopathies. Understanding the underlying pathophysiology (immunology) is essential to formulate treatment strategies.
Immunology
The virus is spread via respiratory droplets and aerosols. The virus then penetrates host cells through endocytosis or membrane fusion.
Viral entry and replication
Coronaviruses have four structural proteins: spike (S), membrane (M), envelop (E), and nucleocapsid (N). The viral surface S protein is required for host attachment and penetration. S1 attaches to the host cell receptor and S2 assists in the fusing of viral and host cell membranes. SARS-CoV-2 binds to the ACE-2 receptor on the host cell membrane, which facilitates viral-host membrane fusion and viral entry. Post entry, SARS-CoV-2 viral RNA gets uncoated and released into the cytoplasm, where it is translated into polyproteins, which are processed by virus-encoded proteinases into individual non-structural proteins (nsps). The (+) strand genomic RNA serves as a template for the replication transcription complex. Following replication, (+) strand genomic RNA is produced which becomes the genome of the new viral particle. The mature virions are discharged from their host cell by exocytosis.
Cytokine storm
Immune response to SARS-CoV-2 is important in determining the clinical course of the infection. In patients with severe COVID-19, SARS-CoV-2 triggers uncontrolled inflammatory responses characterized by elevated pro-inflammatory cytokine production, causing lymphopenia, lymphocyte dysfunction, and granulocyte and monocyte abnormalities. Dysregulated immunological responses result in uncontrollable activation and multiplication of immune cells, lymphocytes, and macrophages, resulting in a cytokine storm. The proinflammatory cytokines IL-1, IL-6, IL-18, IFN-Y, and TNF-α are implicated in cytokine storm. The common laboratory abnormalities which are available to monitor such a response is an altered neutrophil: lymphocyte ratio and highly raised C-reactive protein (CRP).
Interferonopathy
Interferons (IFN) are cytokines that help fight viruses. SARS-CoV-2 blocks interferon regulatory transcription factor, IRF3 nuclear activation and translocation, enabling the coronavirus to multiply unhindered. After binding to interferon gamma receptor (IFNGR), IFN promotes the transcription of IFN-stimulated genes and proinflammatory cytokines/chemokines via activating Janus kinase ½ (JAK1/2). The virus may avoid interferon-mediated immune attack by capping its mRNAs. The SARS-CoV-2 nucleocapsid protein also suppresses IFN induction. Some patients produce autoantibodies against type 1 interferons, predisposing them to severe COVID.
All these mechanisms interact to promote uncontrolled viral replication. Persistent antigenemia will increase macrophage recruitment, culminating in a cytokine storm.
Inflammasome activation
Inflammasomes are large protein complexes that develop in response to Pathogen-associated molecular pattern molecules (PAMPs) or Damage-associated molecular patterns (DAMPs). Inflammasomes are activated by monocytes, macrophages, and barrier epithelial cells.
Inflammasome activation causes severe COVID-19 disease. IL-1 is released early in SARS-CoV-2 infection, whereas type I interferons are suppressed. Many risk factors for severe illness are linked to increased baseline IL-1 production (such as obesity, heart disease, hypertension). Reduced type I interferon production is linked to ageing, interferon pathway mutations, and autoantibodies.
The inflammasome is triggered by two methods: viral intrinsic mechanisms that activate NLRP3 (NOD-, LRR- and pyrin domain-containing protein 3) and host intrinsic mechanisms that activate AIM2(Absent In Melanoma-2). These two events activate caspase 1, which cleaves gasdermin-D(GSDMD) into amino- and carboxy-terminal (NT) fragments. The GSDMD NT fragment binds to the plasma membrane, oligomerizes and inserts itself as a pore. Caspase 1 also cleaves pro-IL-1β and pro-IL-18 into their mature forms, which are released through the GSDMD pore. Toxins like IL-6 may be released by macrophages when exposed to IFN-γ. This leads to the release of lactate dehydrogenase and the formation of extracellular vesicles rich in tissue factors.
Thrombo-inflammation
Although pneumonia is the main manifestation of COVID-19, the emergence of severe disease due to non-pulmonary organ damage in the shadow of coagulopathy often leads to severe disease. Due to the strong link between the inflammatory system and coagulation, COVID-associated coagulopathy (CAC) pathophysiology is complex. Pro-inflammatory cytokines, neutrophils, the complement system, and a shift in the fibrinolytic system are all involved. The pathophysiology of CAC requires aberrant platelet function, especially endothelial dysfunction. Further, COVID-19 has a range of cardiovascular effects.
Immunology of vaccine
Vaccines for COVID-19 have been developed at a record pace utilizing various platforms. The inactivated virus and adenoviral vector derived vaccines available in India at present have been demonstrated to be effective in RCT as well as in real-world setting. A deeper understanding of the immunology with natural infection and vaccines have emerged recently as mentioned below:
- Natural infection provides greater protection than vaccination.
- Booster doses have been demonstrated to be immunogenic.
- Recent research suggests that boosting with a heterologous vaccination are better than homologous boosting in terms of immune response.
Nasal booster doses and pan-COVID vaccination development are current areas of thrust in research.
Therapeutics (Box 1 and Fig. 1)
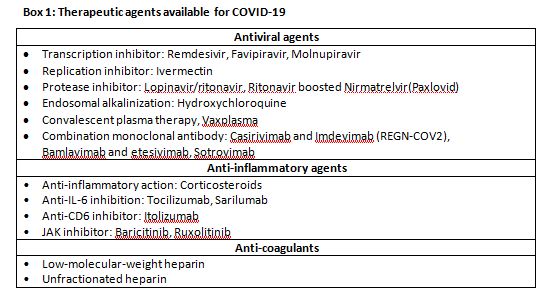
Antiviral drugs
Remdesivir
Remdesivir is an antiviral mono-phosphoramidate prodrug. The active component is an adenosine analogue that inhibits the RNA dependant RNA polymerase(RdRp) enzyme responsible for adding nucleotides to viral RNA strands. By interfering with viral RdRp activity and prematurely terminating RNA chains, it inhibits viral RNA synthesis and replication. The ACTT-1 study showed that remdesivir was superior to placebo in terms of reducing the time to recovery in people hospitalized with COVID-19 and signs of lower respiratory tract infection. Additionally, subgroup analysis revealed a mortality advantage in patients receiving low-flow oxygen. However, comparable results were not seen in other randomised controlled trials (RCTs), including the WHO led SOLIDARITY trial.
Molnupiravir
Molnupiravir is a ribonucleoside prodrug with activity against the SARS-CoV-2. RdRp uses active form of the drug, D-N4-hydroxycytidine (NHC) triphosphate, as a substrate rather than cytidine triphosphate or uridine triphosphate. When the RdRp uses the generated RNA as a template, NHC commands G or A to be included. Researchers believe NHC may create stable base pairs with G or A in the RdRp active core, explaining how the polymerase avoids proofreading and synthesizes changed RNA. However, molnupiravir has been linked to mutagenesis and teratogenicity in the host. Molnupiravir reduces the risk of hospitalization for any cause through day 29 in non-vaccinated at-risk people with Covid-19. The study group had a 10% probability of requiring hospitalization which was reduced to 7% in the molnupiravir group. Considering low absolute risk of hospitalization with the current variant (omicron) and the side-effect profile, the risk-reward ratio for its use must be carefully weighed.
Ritonavir boosted Nimretavir (Paxlovid)
Nirmatrelvir is a new main protease (Mpro) inhibitor that was developed particularly to prevent the action of the SARS-CoV-2 Mpro, an enzyme required for coronavirus replication. The combination therapy was approved by the FDA in December 2021 for its effectiveness in lowering hospitalization and mortality in unvaccinated patients with a moderate to high risk of progression through day 28. It should be initiated early in the course of the illness, although caution should be used since ritonavir is a powerful cyp 450 3A4 inhibitor and pharmacokinetic enhancer.Currently, it is not available in India.
Monoclonal antibodies
Monoclonal antibodies (mAbs) have emerged as very effective tools for treating a wide variety of illnesses due to their great specificity and dependability. Due to the rapid mutation rate of SARS-CoV-2, which is exacerbated by the selective pressure of aggressively deployed preventive vaccinations and neutralizing Abs, the use of Ab cocktail therapy is predicted to be a critical technique for successful COVID-19 treatment. Two of these antibody combinations, bamlanivimab/etesivimab and casirivimab/imdevimab, as well as sotrovimab monotherapy, have been proven to be efficacious in seronegative individuals with mildCOVID-19 who are at high risk of progression. Sotrovimab has also been demonstrated to be more effective against omicron than other monoclonal antibodies.
Ivermectin
This repurposed antiparasitic drug also has antiviral and anti-inflammatory propertiesat higher doses. It is frequently employed because to its theoretical validity and physiologic-based pharmacokinetic modelling studies (PBPK). Although available clinical trials show lower mortality and requirement for mechanical breathing, the quality of evidence is limited owing to significant risk of bias in multiple studies and substantial imprecision.
Convalescent plasma therapy
Convalescent plasma (CP) contains antibodies that neutralize viruses. Other immunological processes, including as antibody-dependent cellular cytotoxicity, complement activation, or phagocytosis, may contribute to CP therapeutic efficacy in patients with COVID-19. Most studies have demonstrated no benefit with CP treatment in terms of disease progression or death; however, this observation is attributed to the late administration, in the second week of illness. The INFANT-COVID-19 group established the effect of high-titre plasma administered within 3 days of illness start in reducing disease progression. Vax-plasma is CP obtained from individuals who have recovered from a natural infection and have been vaccinated later. It has 10-100 times the antibody titres of regular high-titre CP and covers most known COVID-19 variations. Its usage may be a potential therapy for treating and preventing COVID-19 infection in immunocompromised persons.
Others
Hydroxychloroquine has immunomodulatory and antiviral properties. Many in vitro investigations proved its efficacy against SARS-CoV-2. However, multiple clinical trials found it to be ineffective against COVID-19.
Anti-inflammatory drugs
Corticosteroids
Corticosteroids are considered mainstay of therapy in COVID-19 patients with moderate to severe hyperinflammation. Steroids are used to alleviate inflammation if they are administered at the appropriate time, since premature commencement might boost viral multiplication and delay adaptive immunity. Corticosteroids may be most useful when begun during the pulmonary phase of the disease process because they may mitigate the intensity of inflammation and avoid the severe hyperinflammation phase in COVID-19.The Randomized Evaluation of COVID-19 Therapy (RECOVERY) collaboration revealed that corticosteroid treatment had a significant survival advantage in participants receiving oxygen or mechanical breathing. Corticosteroids act by suppressing the generation of cytokines and limiting the clearance of macrophages and natural killer cells.
IL-6 receptor blockers
As part of the acute-phase response, interleukin-6 is generated in response to infection and promotes inflammatory pathways. Tocilizumab (TCZ) and sarilumab are monoclonal antibodies that block membrane-bound and soluble interleukin-6 receptors. For its use in COVID-19, several early RCTs found no benefit with TCZ, although these trials had flaws. Many of these trials were done in the early stages of the pandemic, but the criteria for recruitment, the timing of TCZ administration and the main outcome of interest were all inconsistent, and only a limited percentage of patients got corticosteroids. Results from RECOVERY & REMAP-CAP trials suggest that treatment with the interleukin-6 receptor antagonists including survival, in critically sick patients receiving organ support in addition to systemic corticosteroids.
There are a few more immunosuppressive agents being studied for the treatment of COVID-19, including anakinra(IL-1 antagonist) and baricitinib (JAK-1 and 2 inhibitor). In hospitalized patients, the ACTT-2 study indicated that the combination of baricitinib and remdesivir reduced the time of recovery by one day when compared to remdesivir alone. Patients needing high-flow oxygen and non-invasive ventilation had a greater magnitude of impact.
Anticoagulation
COVID-19increases the risk of venous thromboembolism in critically ill patients. According to a recent meta-analysis, the risk of arterial thromboembolism (ATE) was 5% and venous thromboembolism (VTE) was 31%among SARS-COV-2 infected patients admitted at ICU. This demonstrates that COVID-19 has increased immuno-thrombosis and hence, thrombo-prophylaxis with anticoagulant therapy is indicated for all hospitalized COVID-19 patients.
The treatment strategies for mild, moderate, and severe COVID-19 are shown in Box 2-4 which reflects the current understanding of the underlying immunology and evidence generated for the available therapeutic options in India.
References
- Vora SM, Lieberman J, Wu H. Inflammasome activation at the crux of severe COVID-19. Nat Rev Immunol. 2021;21(11):694-703.
- Zhang Q, Bastard P, Bolze A, et al. Life-Threatening COVID-19: Defective Interferons Unleash Excessive Inflammation. Med (N Y). 2020;1(1):14-20.
- Yang, L., Liu, S., Liu, J. et al.COVID-19: immunopathogenesis and Immunotherapeutics. Sig Transduct Target Ther 5, 128 (2020).
- Taus F, Salvagno G, Canè S, et al. Platelets Promote Thromboinflammation in SARS-CoV-2 Pneumonia. ArteriosclerThrombVasc Biol. 2020;40(12):2975-2989.
- Beigel JH, Tomashek KM, Dodd LE, ACTT-1 Study Group Members. Remdesivir for the Treatment of Covid-19 – Final Report. N Engl J Med. 2020;383(19):1813-26.
- WHO Solidarity Trial Consortium. Repurposed Antiviral Drugs for Covid-19 – Interim WHO Solidarity Trial Results. N Engl J Med. 2021;384(6):497-511.
- Salama C, Han J, Yau L, Reiss WG, Kramer B, Neidhart JD, et al. Tocilizumab in Patients Hospitalized with Covid-19 Pneumonia. N Engl J Med. 2021;384(1):20-30.
- REMAP-CAP Investigators. Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N Engl J Med. 2021;384(16):1491-502.
- Malas MB, Naazie IN, Elsayed N, et al. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: A systematic review and meta-analysis. EClinicalMedicine. 2020;29:100639. D
- Desai D, Khan AR, Soneja M, et al. Effectiveness of an inactivated virus-based SARS-CoV-2 vaccine, BBV152, in India: a test-negative, case-control study. Lancet Infect Dis. 2021 Nov 23:S1473-3099(21)00674-5.