(Angiotensin Receptor Neprilysin Inhibitor)
https://meditropics.com/?page_id=898&preview=true
*Vanshika Sawhney
*Post Graduate Resident, Department of Medicine, LHMC
INTRODUCTION:
Heart failure is defined as complex clinical syndrome that results from structural or functional impairment of ventricular filling or ejection of blood, which in turn leads to cardinal clinical symptoms of dyspnea and fatigue and signs of heart failure, namely edema and rales. (1)
Neurohormonal pathways are thought to be fundamentally important in the pathophysiology of heart failure. It is known that sustained activation of certain neurohumoral pathways such as the renin –angiotensin –aldosterone system (RAAS) and sympathetic nervous system (SNS) is detrimental in heart failure.
Although the focus of therapeutic intervention has been on blocking these pathways thought to be harmful in heart failure, potentially beneficial counter-regulatory systems are also activated in heart failure. These pathways variously promote vasodilatation and natriuresis, inhibit abnormal growth, suppress the RAAS and SNS, inhibit the release and actions of vasopressin, and augment the parasympathetic nervous system.
The best understood mediators exerting these actions are the natriuretic peptides. The first of these to be described, A-type natriuretic peptide (ANP), is secreted in response to atrial distension, activates the ANPR-A receptor, increasing intracellular cyclic guanylate monophosphate (cGMP), and is cleared by the ANPR-C receptor and by the action of the enzyme neutral endopeptidase (NEP), also known as neprilysin. B-type natriuretic peptide, secreted predominantly by the ventricles in response to increased wall stress, exerts similar actions and is cleared in the same way. (3)
The introduction of LCZ696, an ARB (valsartan) with an endopeptidase inhibitor (sacubitril), has shown a survival benefit in a large trial versus ARB alone. The drug, referred to as an angiotensin receptor-neprilysin inhibitor (ARNI) (and denoted Entrezto), was tested in the PARADIGM-HF trial as an alternate to optimally dosed ACEI and demonstrated an incremental improvement in survival when compared to ACEI alone.(2)
MECHANISM OF ACTION:
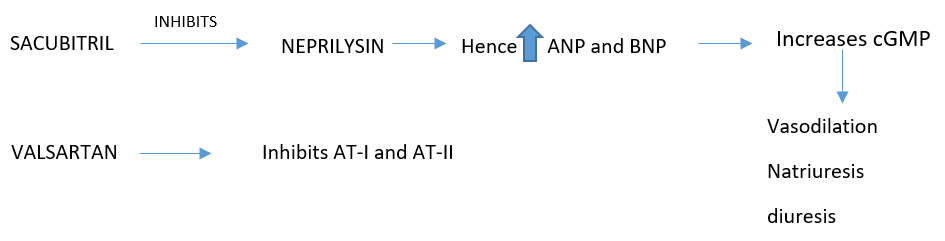
PHARMACOKINETICS:
Absorption – time to peak – 0.5 hours.
Time to peak of metabolite – 2 hours.
Distribution – protein binding – 94-97%
Vd : 103 L
Metabolism – metabolized by esterases to active metabolite. Major metabolite is not metabolized.
Excretion– T1/2 Sacubitril: 1.4 hours
T1/2 Valsartan- 9.9 hours
T1/2 LBQ657- 11.5 hours
INDICATION:
Most guidelines now advocate switching ACEI to this drug as a standard in patients with mild-moderate systolic heart failure when they remain symptomatic despite fully tolerated doses of conventional therapy.
As per 2022 AHA/ACC/HFSA(3) guideline for the management of heart failure–
- For HF with reduced ejection fraction (HFrEF): strong recommendations (Class of Recommendation 1) are made for ARNI
- For HF with mildly reduced ejection fraction (HFmrEF): Weaker recommendations (Class of Recommendation 2b) are made for ARNI
- New recommendations for HF with preserved ejection fraction (HFpEF) are made for ARNI (Class of Recommendation 2b).
DOSING:
- Recommended starting dose is 49/51 mg (sacubitril/ valsartan) twice daily.
- Target maintenance dose: After 2-4 weeks, double the dose to target maintenance dose of 97mg/103mg PO BID as tolerated.
DOSAGE MODIFICATIONS:
Patients not taking an ACE inhibitor or other ARB, or previously taking a low dose of these agents when initiating treatment:
Reduce the starting dose to 24 mg/ 26 mg BID.
Double the dose every 2-4 weeks to target maintenance dose of 97mg/103 mg BID as tolerated.
Renal impairment:
- Mild – to – moderate (eGFR > 30 mL/min/1.73m2 ): no starting dose adjustment required.
- Severe (eGFR < 30 mL/min/ 1.73m2): reduce starting dose to 24mg/26mg BID; double the dose to target maintenance dose of 97mg/103mg PO BID as tolerated.
Hepatic impairment:
Mild– no starting dose adjustment required.
Moderate– reduce starting dose to 24mg/26mg BID; double the dose to target maintenance dose of 97mg/103mg PO BID as tolerated.
Severe– not recommended.
ADVERSE DRUG REACTIONS:
- Hypotension
- Hyperkalaemia
- Cough
- Dizziness
- Orthostasis
- Falls
- Angioedema, all patients (0.5%); in black patients (2.4%)
- Hypersensitivity
- Increases creatinine.
CONTRAINDICATIONS:
- Hypersensitivity to any component.
- History of angioedema related to previous ACE inhibitor or ARB therapy.
- Concomitant use with ACE inhibitors. Do not administer ACE inhibitors within 36 hours of switching to or from sacubitril/ valsartan.
- Concomitant use with aliskiren in patients with diabetes.
- Not recommended in patients with severe hepatic impairement.
CAUTIONS:
- Can cause foetal harm when administered to a pregnant woman.
- Monitor renal function and potassium levels in susceptible patients (eg diabetes, hypoaldosteronism, high potassium diet, renal artery stenosis) (4)
REFERENCES:
- Givertz MM, Mehra MR “Heart Failure: Pathophysiology and Diagnosis.” Chapter 257 Harrison’s Principles of Internal Medicine, 21e Eds. New York: Joseph Loscalzo, et al. McGraw Hill, 2022, p.1930-1940
- Desai AS, Mehra MR “Heart Failure: Management.” Chapter 258 Harrison’s Principles of Internal Medicine, 21e Eds. New York: Joseph Loscalzo, et al. McGraw Hill, 2022, p.1940-1954
- Writing Committee Members; ACC/AHA Joint Committee Members. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure. J Card Fail. 2022;28(5): e1-e167.
- McMurray JJ, Packer M, Desai AS, et al. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur J Heart Fail. 2013;15(9):1062-10