https://meditropics.com/527-2/ Parmita Barua Consultant, Hepatology Clinic & Department of Medicine,ABVIMS & Dr.RML Hospital, New Delhi
Hepatitis B
- HBV is a small enveloped DNA virus belongs to the family Hepadnaviridae.
- HBV is 50-100 times as infectious as HIV and 10 times as infectious as HCV.
- The genome is composed of 4 open reading frames (Figure-1)
- Core gene – pre-C and core region- Hbe& HBc protein
- Surface gene – HbS antigen
- X gene—HbX protein which has transactivating and oncogenic properties.
- Pol gene – polymerase
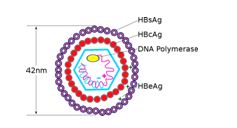
Figure-1
Epidemiology
- India falls into the intermediate (2-8%) prevalence of Hepatitis B. There are approximately 10 genotypes of HBV across the world. Genotype D isprevalent in India.
Routes of HBV Transmission
- Parenteral, Perinatal, Sexual Route and by close person to person contact (by open cuts and sores).
- In India (intermediate prevalence) perinatal route is the most common route of transmission.
- Perinatal Route- If mother positive for HBsAg and HBeAg
- 70%-90% of infants infected
- 90% of infected infants become chronically infected
- If mother is positive for HBsAg only
- 10% of infants infected
- 90% of infected infants become chronically infected Outcome of Chronic HBV infection
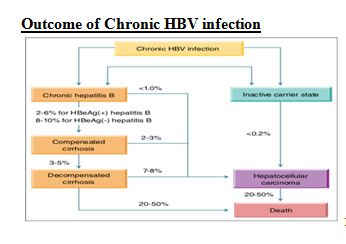
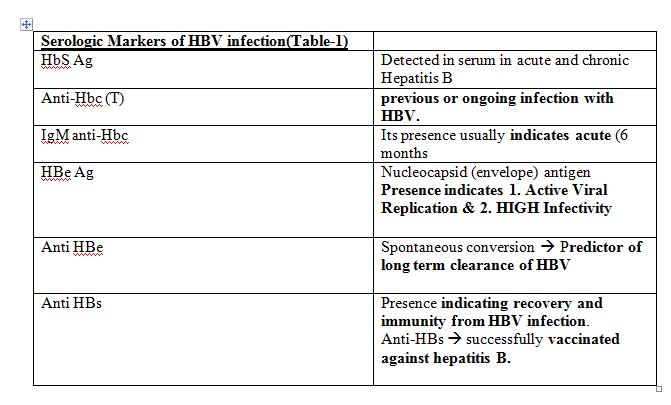
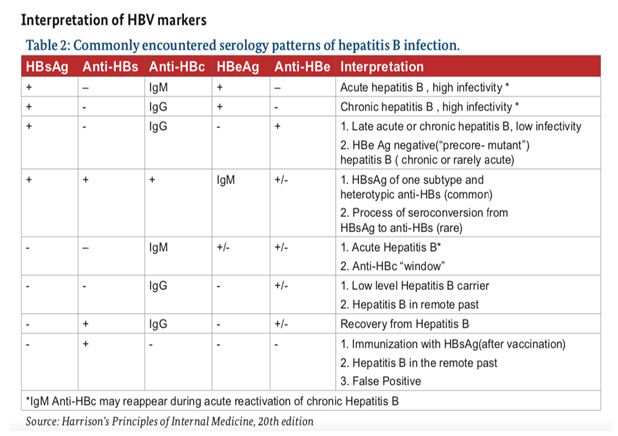
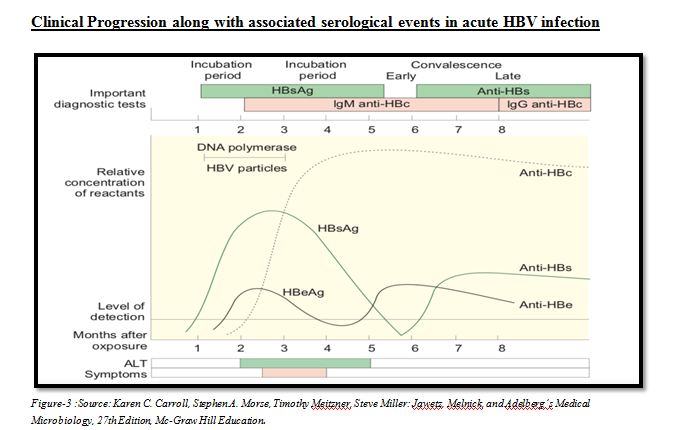
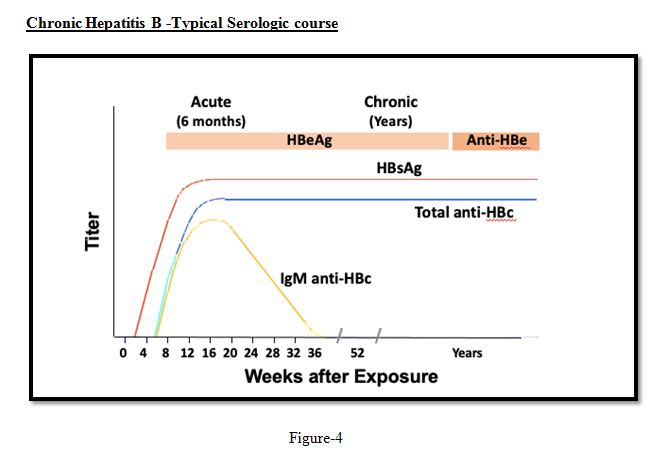
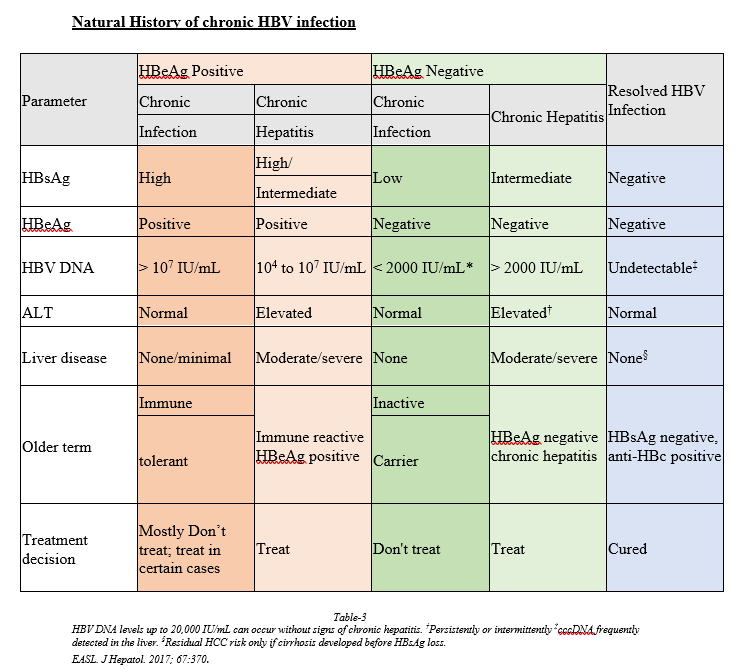
Assessment and Staging of HBV Chronic infection
- Serological markers of HBV infection ;
- Measurement of HBV DNA levels; and
- Assessing severity of liver disease by
- Liver enzymes
- Non-invasive tests (NITs) such as aspartate aminotransferase (AST)-to-platelet ratio index (APRI), FIB-4, transient elastography (FibroScan).
- Liver biopsy, if available
Goals of Therapy
- To improve Liver Histology
- Normalization of serum ALT
- Seroconversion (HBe Ag loss, anti-HBe production, HbS antigen loss )
- Normalization of ALT
- Decline of serum HBV DNA
- Prevention of cirrhosis, HCC, and death.
Whom to Treat


Pharmacotherapy of HBV
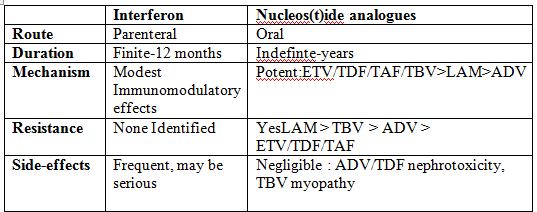
- INF- ⍺& Peg-INF-⍺
- Nucleoside analogues – Entecavir, Tenofovir disoproxil fumarate, Tenofovir Alafenamide, Adefovir, Lamivudine, Telbivudine.
Selection of antiviral drug for CHB
- In all adults, adolescents and children aged 12 years or older in whom antiviral therapy is indicated, the NAs which have a high barrier to drug resistance (tenofovir or entecavir) are recommended.
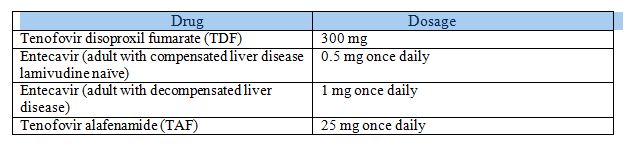
- In patients with compensated cirrhosis or CHBV antiviral therapy recommended are Entecavir (0.5 mg),Tenofovir Disoproxil Fumarate (300 mg), Tenofovir Alafenamide (25mg),Peg IFN.
- In patients with decompensated cirrhosis Entecavir 1 mg and TDF 300 mg recommended.
- Peg-IFN is contraindicated in decompensated cirrhosis.
- In woman of childbearing age Tenofovir may be preferred as the drug of choice in the eventuality of a pregnancy. Entecavir is not recommended in pregnancy.
- Tenofovir is preferred in patients who have been exposed to lamivudine who have a potential for Entecavir resistance.
- Entecavir is recommended in children aged 2–11 years.
- Entecavir may be preferred over Tenofovir in:
- Age > 60 years; bone disease due to chronic steroid use or use of other medications that worsen bone density, history of fragility fracture, osteoporosis; altered renal function with eGFR < 60 mL/min/1.73 m2 or albuminuria > 30 mg/ 24 hr or in patient on hemodialysis (Ref: EASL guidelines)
- Tenofovir alafenamide fumarate ( TAF) is the drug of choice in patients with reduced renal function or bone disease bone toxicities, where entecavir is contraindicated
- Drugs with a low barrier to resistance (lamivudine, adefovir or telbivudine) are available but not recommended as they lead to drug resistance.
Approved Doses for Patients with Renal Impairment
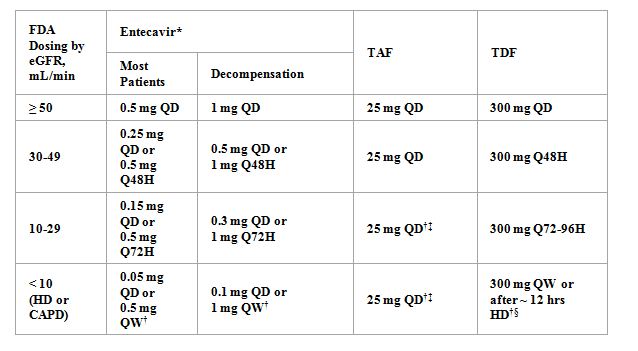
When To Stop Treatment
- PegIFN: defined duration of 48 wks; contraindicated in patients with decompensated cirrhosis
- Nucleos(t)ide analogues: endpoint depends on cirrhosis and/or HBeAg status
- Cirrhosis: indefinite antiviral therapy (unless strong competing rationale for stop)
- HBeAg positive: consider discontinuation following seroconversion to anti-HBe + consolidation therapy for ≥ 12 mos; check HBV DNA, ALT, seroconversion, and decompensation every 3 mos in first yr after cessation
- HBeAg seroconversion: ~ 50% after 5 yrs of treatment
- HBeAg negative: indefinite antiviral therapy; if considering discontinuation in patients with mild disease, discuss risks and defer to center with expertise
- HBsAg loss: ~ 5% after 5 yrs of treatment
HBV Management in Patients Undergoing Immunosuppression
Screen for HBsAg and anti-HBc (total or IgG) before initiation of immunosuppressive, cytotoxic, or immunomodulatory therapy
- If HBsAg(+) and anti-HBc(+), give prophylactic NA therapy
- If HBsAg(-) and anti-HBc(+), either give prophylactic NA therapy or monitor ALT, HBV DNA, and HBsAg
- If monitoring chosen, measure HBV DNA every 1-3 mos; treat with an NA upon first sign of reactivation (ie, HBV DNA elevation, HBsAg seroreversion)
- Patients receiving anti-CD20 therapy (eg, rituximab) or undergoing stem cell transplantation should receive anti-HBV prophylaxis.
Preferred prophylaxis: ETV, TAF, or TDF during and for 6-12 mos after immunosuppressive therapy.
HBV and Pregnancy
Hbs Ag should be checked in all pregnant women.
Women who are Hbs antigen positive should be evaluated further with ALT, HBV DNA, or imaging for HCC , women who meet the indications should be treated with antiviral therapy.
- Women without standard indications for HBV therapy but HBV DNA >2,00,000 IU/ml in the second trimester should consider treatment to prevent mother to child transmission.
Antiviral therapy to be started at 28-32 weeks of gestation.
TDF, Lamivudine and Telbivudine. TDF preferred choice.
Duration of antiviral therapy –Antiviral therapy to be discontinued at the time of delivery or upto 4 weeks postpartum.
If Mother Hbs Antigen positive – Hepatitis B immune globulin and HBV vaccine should be administered to the newborn < 12 hours after delivery.
If Hbs antigen negative –HBV vaccination may be considered in women who are not previously vaccinated and it is both safe and efficacious during pregnancy.
Breastfeeding is not prohibited.
HBV infected pregnant women with cirrhosis should be managed in high risk obstetrical practices and treated with TDF to prevent decompensation.
C-section is not indicated owing to insufficient data.
HBV & HIV co-infection
All patients with HBV & HIV co-infection should receive ARVT that includes 2 drugs with activity against HBV: specifically (TDF or TAF) plus lamivudine or Emtricitabine.
Pts with co-infection should initiate ARVT irrespective of CD4 count.
TAF is approved for HIV in combination with emtricitabine with or without other HIV drugs and is preferred to TDF because of its improved safety profile.
Hepatitis flares may occur during the first few weeks of treatment from immune reconstitution.
Hepatitis C
Hepatitis C virus initially called as non-A, non-B virus, single-stranded enveloped positive sense RNA virus belonging to the Flaviviridae family.
Clinical Course of Hepatitis C Infection
- Hepatitis C infection is usually acquired through infected syringes and needles, and transfusion of infected blood.
- Sexual transmission of HCV occurs infrequently in heterosexual couples.
- It is reported to be more common in HIV-positive persons.
- The risk of transmission of HCV from a mother to her child occurs in 4–8% of births to women with HCV infection, and in 10.8–25% of births to women with HIV and HCV co-infection.
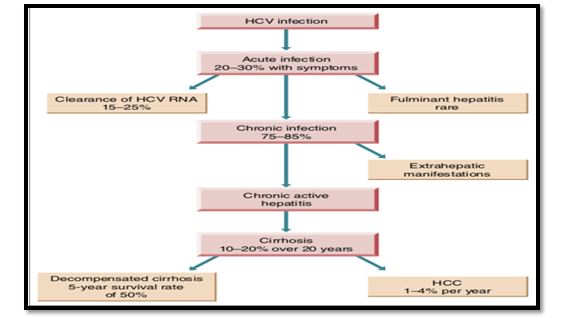
- HCV causes both acute and chronic hepatitis.
- Acute hepatitis is often clinically mild and marked by fluctuating elevations of serum aminotransferase levels;
- >50% likelihood of chronicity, leading to cirrhosis in >20%.
- Chronic infection with HCV is usually clinically silent.
Epidemiology of HCV in India
Population prevalence of chronic HCV infection is around 1%.However there are pockets of areas where prevalence of HCV has been observed to be relatively higher in Punjab, Haryana, Andhra Pradesh, Puducherry, Arunachal Pradesh, Mizoram. Specifically in higher risk group like injection drug users, truck drivers, and attendees of STI infection clinic.
Laboratory Diagnosis
- Following an initial eclipse phase of 1–2 weeks when no virological or serological markers of infection may be detected, the natural course of HCV infection is characterized by the appearance of HCV RNA, then HCV core p22 Ag in the absence of an antibody response for a further 6–10 weeks.
- During this serological window, it has been shown that free (i.e. not complexed with antibody) HCV core antigen (HCVcAg) can be detected in a proportion of individuals.
- Following the development of the antibody response, HCVcAg becomes complexed with these antibodies specific for HCV.
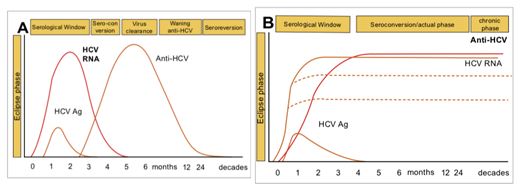
The standard method of diagnosis is by detection of anti-HCV antibody. Since anti-HCV antibodies from an infected mother may persist in children <18 months of age, HCV RNA detection is also used to diagnose HCV infection in this age group (after 2 months of age) .
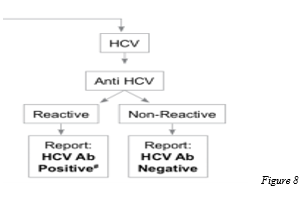
Assessment of Degree of Fibrosis
- Clinical evaluation for features of cirrhosis and evidence of decompensation, and
- Measurement of serum bilirubin, albumin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and prothrombin time; as well as full blood count, including platelet count.
Other routine investigations include ultrasonography and alpha-fetoprotein (AFP).
Non-invasive tests (NITs):
- Non-invasive methods for assessing the stage of liver disease are validated in adults with CHCV.
- APRI (AST-to-platelet ratio index) and FIB 4 are recommended as the preferred non-invasive tests (NIT) to assess for the presence of cirrhosis (APRI score >2: FIB 4 >3.25 in adults).
- Transient elastography (e.g. FibroScan) may be the preferred NITs.


Whom To Treat ?
- Any individual diagnosed to have infection with hepatitis C virus (viremia +) needs treatment.
- The duration of treatment will depend on the several situations such as, cirrhosis versus non-cirrhosis, presence of decompensation (ascites, variceal bleeding, hepatic encephalopathy, or infection(s), treatment naïve versus treatment experienced (to peg IFN, DAAs, etc).
Pharmacotherapy for HCV
- NS3 Protease Inhibitors(-previrs):Grazoprevir (GZR) Paritaprevir/Ritonavir(PTV/RTV)
Simeprevir (SMV)
Voxilaprevir (VOX)
Glecaprevir (GLE)
- NS5A Replication Complex Inhibitors(-asvir):Daclatasvir (DCV)
Elbasvir (EBR)
Ledipasvir (LDV)
Ombitasvir (OBV)
Velpatasvir (VEL)
Pibrentasvir (PIB)
- NS5BNUC Inhibitors(-buvir):Sofosbuvir (SOF)
- NS5 Non-NUC Inhibitors(-buvir):Dasabuvir (DSV)
What regimen to use ?
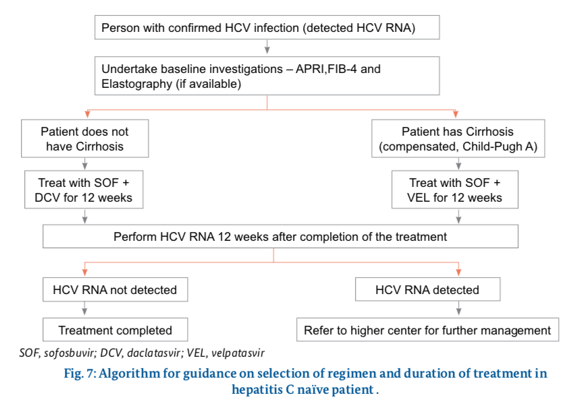
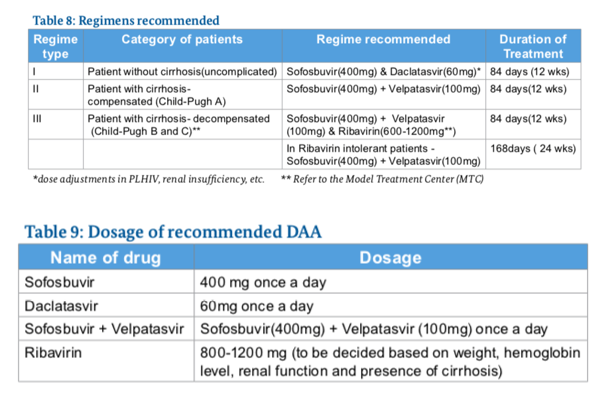
Side Effects of Drugs Used in Treatment
- New DAA regimens are well tolerated by patients.
- Certain regimens have been shown to be safe for use in patients with decompensated liver cirrhosis and those who have undergone liver transplantation.
- Daclatasvir: fatigue, headache and nausea.
- Sofosbuvir with or without ledipasvir: Fatigue, headache, insomnia and nausea.
- Significant bradyarrhythmias are reported with sofosbuvir in patients also taking amiodarone and therefore it is contraindicated in these patients.
- Sofosbuvir with Velpatasvir : Headache, fatigue, anemia, nausea, insomnia, diarrhea, weakness, rash and depression are the most common adverse events reported .
Ribavarin
- Anaemia is a common, predictable side-effect of ribavirin therapy
- Patients whose haemoglobin level falls below 10 g/dL should have their ribavirin dose reduced from 800–1200 mg/day (depending on the patient’s weight and HCV genotype) to 600 mg/day.
- A patient whose haemoglobin level falls below 8.5 g/dL should discontinue ribavirin therapy.
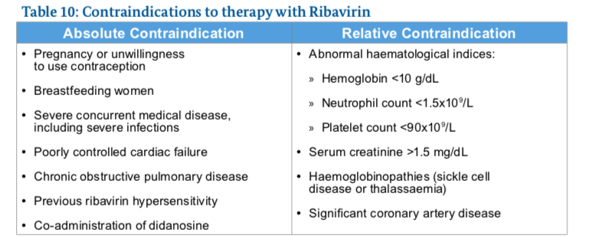
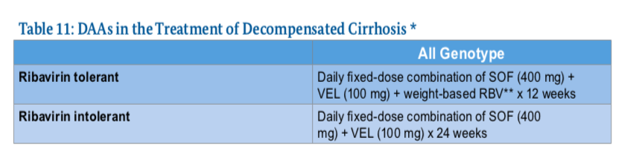
DAAs in the treatment of decompensated cirrhosis:
DAAs can cause severe complications when prescribed to persons with decompensated cirrhosis .
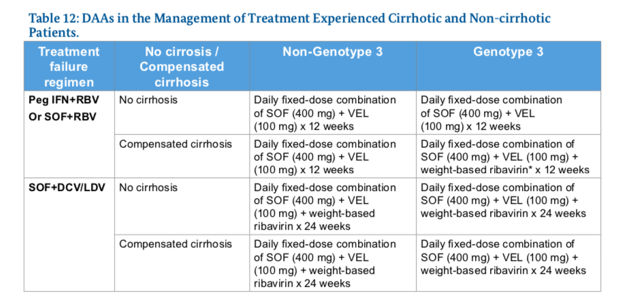
Management of Treatment Experienced Patients
Management of Cirrhotic Patients after HCV clearance in SVR 12
- HCV infection can be considered cured in non-cirrhotic patients who have achieved a SVR 12 after 12 weeks of completing the treatment.
- Thus no follow-up is required.
- HCC surveillance in these patients must be indefinite.
Persons with HIV/HCV Co-infection
- Persons with HIV/HCV co-infection generally have more rapid progression of liver fibrosis, especially those with a CD4 cell count of <200 cells/mm3.
- For these reasons, all persons with HIV/HCV co-infection should be considered for HCV treatment.
- It is advisable to first initiate treatment for HIV and achieve HIV suppression before starting HCV treatment.
- ALT and AST should be monitored at 1 month after ART initiation and then every 3–6 months.
- For most HIV/HCV co-infected persons, including those with cirrhosis, the benefits of ART outweigh concerns regarding drug-induced liver injury.
- Daclatasvir is associated with significant drug interactions with many NNRTIs and PIs.
- The dose of daclatasvir will be 30 mg with ATV/r and 90 mg with EFV.
- Ledipasvir (LDV) and sofosbuvir have shown reduced potential for drug interactions with ARV drugs due to their use of different metabolic pathways .
Persons with HBV/HCV co-infection
- HBV and HCV co-infection may result in an accelerated disease course; HCV is considered to be the main driver of disease.
- During treatment and after HCV clearance, there is a risk of reactivation of HBV, and this may require treatment with concurrent anti-HBV antiviral therapy.
Persons with TB/HCV co-infection
- People at increased risk of infection with HCV are also often at increased risk of infection with TB.
- Most of the DAAs interact with metabolic pathways in the liver, which increases and/or decreases the drug level of DAA .
- Therefore, concurrent treatment of HCV infection and TB should be avoided.
- Active TB should generally be treated before commencing therapy for HCV.
Women of child-bearing age
- Thus, women with childbearing potential should be counselled that they require effective contraception during treatment and for six months after completion of therapy.
- Safety of DAAs in pregnancy has not been established .
- DAAs are thus contraindicated in pregnant women and those with child bearing potential for 6 months after completing therapy.