INSULIN THERAPY IN TYPE 2 DIABETES MELLITUS
https://meditropics.com/637-2/
Apoorva Ojha
Post graduate resident, department of medicine, LHMC
INSULIN BIOSYNTHESIS
Insulin, produced by the beta cells of the pancreatic islets, is initially synthesised as a single-chain 86-amino-acid precursor polypeptide, preproinsulin. Removal of the amino-terminal signal peptide, gives rise to proinsulin. Proinsulin is structurally related to IGF I and II, which bind weakly to the insulin receptor.
Cleavage of an internal 31-residue fragment from proinsulin generates C-peptide with the A chain(21 amino acids) and B chain(30 amino acids) of insulin being connected by disulfide bonds. The mature insulin molecule and C-peptide are stored together in the beta cells.
INSULIN SECRETION
Insulin is secreted in a pulsatile manner at 4-15mins of duration. Glucose levels >70 mg/dL stimulate insulin synthesis. Insulin secretion is characterised by continuous basal secretion with peaks occurring soon after meals.
Basal insulin secretion: amount of insulin secreted in the fasting state in the absence of exogenous stimuli which accounts to be 50%.
Stimulated insulin secretion, comprising remaining 50%, occurs in response to exogenous stimuli such as ingested meals.
Glucose is the most potent stimulant of insulin release. When the glucose concentration in an individual is increased suddenly, an initial short-lived burst of insulin release occurs (first-phase insulin response). The first phase lasts for 10mins. If the glucose concentration is held at this level, the insulin release gradually falls off and then begins to rise again to a steady level (second-phase insulin response). The second phase of insulin secretion lasts for 80-150mins.
Total insulin produced by a lean, healthy individual is between 18-40 U/day or 0.2–0.5 U/kg/day. 50% of total insulin is basal insulin and secreted at rate of 0.5–1.0 U/h.

Fig: Endogenous insulin secretion
REGULATION OF GLUCOSE HOMEOSTASIS
Glucose homeostasis is the balance between energy intake from ingested food, hepatic glucose production (gluconeogenesis) and peripheral tissue glucose uptake and utilisation. In fasting state: low insulin and increased glucagon leads to increased glucose production. The fasting state leads to hepatic gluconeogenesis, glycogenolysis and reduced glucose uptake in peripheral tissues (skeletal muscle and fat) and lipolysis. Postprandially, the glucose load elicits a rise in insulin and fall in glucagon, leading to a reversal of these processes. Its important to note that brain utilises glucose in an insulin independent manner.
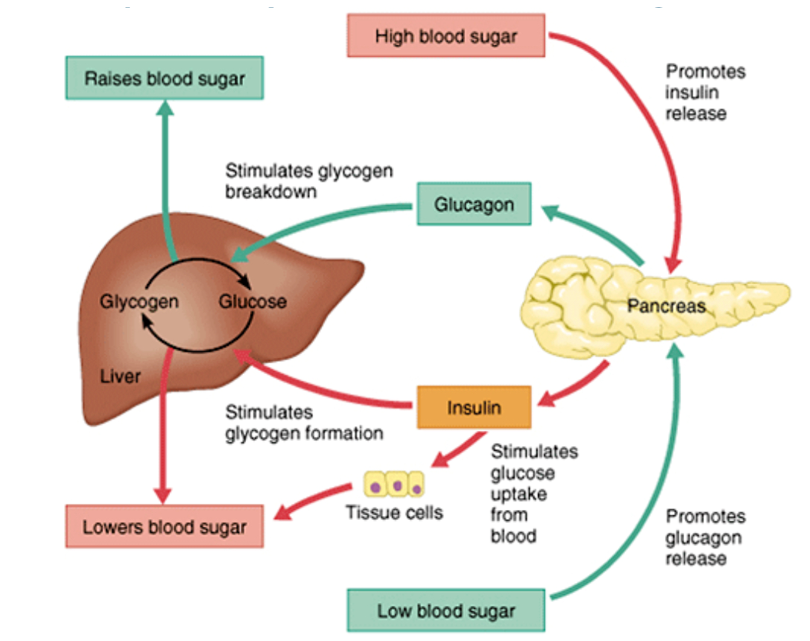
Fig: Glucose Homeostasis
INSULIN PREPARATIONS

Earlier Bovine and Porcine based insulins were available; but were highly immunogenic. This led to development of recombinant DNA technology based insulin.
Two types of insulin commercially available:
- Human insulin (NPH & Regular=have same amino acid sequence as native insulin)
- Insulin analogues

Insulin Icodec: once weekly insulin under research trial. It reversibly binds to albumin and is released as depot. Loading dose might be required as steady state takes time to reach.
Rapid acting insulin analogues
It includes Lispro, aspart, glulisine.
Lispro: 28th and 29th amino acid (lysine and proline) are reversed in B chain.
Aspart: proline is substituted with aspartic acid at 28th position in B chain.
Glulisine: amino acid asparagine at position B3 is replaced by lysine and the lysine in position B29 is replaced by glutamic acid.
The insulin preparations have got full biologic activity, low tendency of self aggregation; hence rapid onset and shorter duration of action. They are advantageous against rising plasma glucose levels following meals (bolus regimen). This renders to less hypoglycemia episodes and hence preferred over Regular Insulin for prandial coverage in many patients. These are available as 100 IU/ml preparations.
SHORT AND INTERMEDIATE ACTING INSULIN
Human Insulin: Regular(short acting/soluble) & NPH(intermediate acting/insoluble). Both are available from 40-100 IU/ml preparations. A preparation of regular insulin -500IU/ml is also commercially available for severe insulin resistance.
NPH: used as basal insulin but is not an ideal basal insulin as it shows much variability in its absorption and action after subcutaneous injection. NPH insulin is obtained from the precipitation of recombinant synthesized human insulin with zinc in the presence of protamine, a basic polyarginine peptide. It is isophane suspension of human insulin.
Adverse effects of NPH insulin:
- Nocturnal hypoglycemia (if taken before dinner- peak occurs at midnight when requirement is much less).
- Fasting hyperglycemia: if taken before dinner- duration of action is not enough to maintain glucose homeostasis till morning; can be taken at bedtime to minimise this effect.
Ideal Basal Insulin properties:
- Square-wave action profile, ie, no peak, long-lasting
- Reproducible effects (after several injections)
- Pharmacodynamic effects similar to pump insulin
In T2DM; endogenous insulin secretion continues and is capable of covering prandial rise of glucose and hence a single long acting insulin (Glargine) is preferred for initiating insulin therapy.
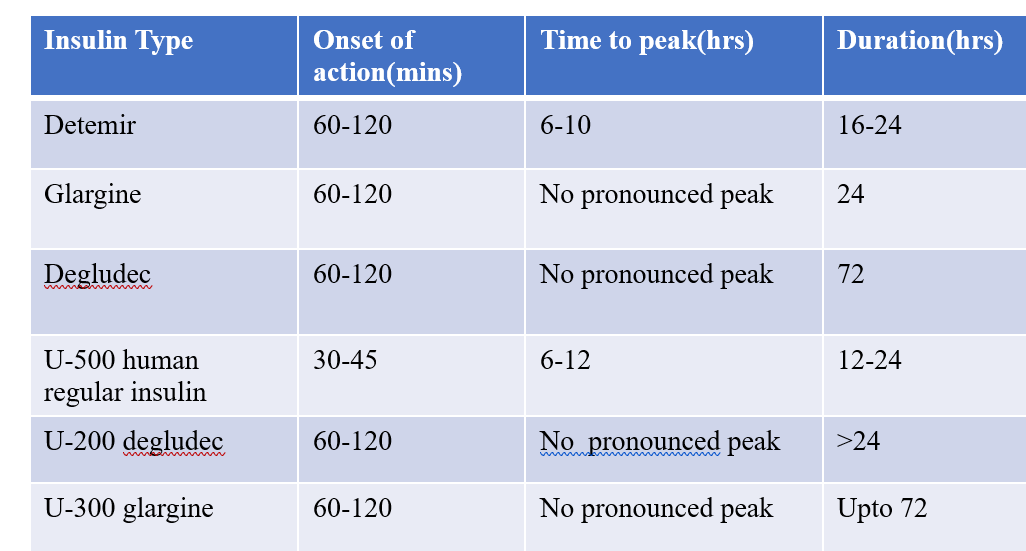
LONG ACTING INSULIN: PROPERTIES
Detemir: has fatty acid side chain; reversibly binds to albumin- hence slow absorption and catabolism.
Glargine: glycine in place of asparagine at 21st position + 2 arginine residues at C terminal of B chain leading to formation of microprecipitates at injection site due to change of pH. Degludec (ULTRA LONG ACTING): modification and extension of C terminal of B chain; forms multihexamers at injection site and binds to albumin.
Preparations: U 100,200,300/ml
INHALATIONAL INSULIN
Insulin in powdered form is delivered to the lungs with an inhaler.
Exubera(Pfizer) & Afrezza(Mankind) were the two preparations initially available.
Afrezza: Insulin dissolved with powder; once inhaled, technosphere insulin is rapidly absorbed upon contact with lung surface; thumb size inhaler with a rather increased dosing flexibility.
Exubera failed in trials: in comparison to subcutaneous route, higher dose required due to inefficient absorption. Administration involved use of a bulky device with little dosing flexibility.
Side effects of inhalational insulin:
Prior to its use, the forced expiratory volume in one second (FEV1) should be measured. Can cause bronchospasm and cough and should be not be in individuals with lung disease or who smoke.
COMPLICATIONS OF INSULIN THERAPY
- Hypoglycemia
- Insulin allergy: local or systemic urticaria is due to histamine release from tissue mast cells sensitized by adherence of anti-insulin IgE antibodies. In severe cases, anaphylaxis results. Rare with human insulin and insulin analogues.
- Immune insulin resistance: due to circulating IgG anti-insulin Rare with human insulin, not seen with insulin analogues.
- Lipodystrophy: atrophy- due to insulin immune reaction; rare with human insulin and analogues.
- Hypertrophy– due to insulin accumulation, can be seen commonly, rotation of injection site is must!
RATIONALE ON USING INSULIN IN TYPE 2 DIABETES MELLITUS
Chronic hyperglycaemia and elevated free fatty acids exert harmful effects on β-cell function and regeneration, as well as on the metabolic memory.Early insulinisation can delay or reverse residual β-cell function and loss, respectively. The majority of patients with type 2 diabetes are multimorbid with diabetes-related complications. Seniors with frailty and sarcopenia as comorbidities, and subtypes with severe insulin deficit are candidates for patient-centred timely insulin treatment. Timely initiation of insulin promotes better treatment to target glucose control with lower insulin dosage, lower rates of adverse events, and is cheaper.

INITIATING TREATMENT FOR DM
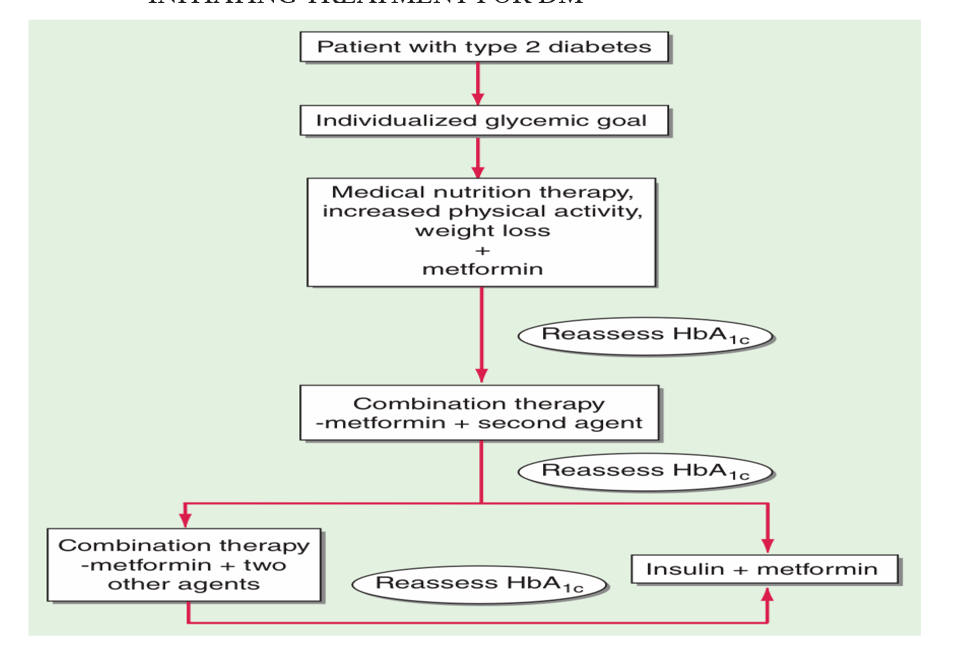
Initiating therapy depends on presence of comorbidities (eg. SGLT2 inhibitors/GLP RA in case of cardiovascular diseases). Usually initiating therapy is Metformin unless C/I. Assessment has to be done every 3 months to check if the glycemic levels are within the target range. When A1C is ≥1.5% above the glycemic target, combination therapy needs to be started. (OHAs+ Insulin or 2 OHAs of different class). Metformin should be continued upon initiation of insulin therapy (unless contraindicated or not tolerated) for ongoing glycemic and metabolic benefits. In patients with type 2 diabetes, a glucagon-like peptide 1 receptor agonist is preferred to insulin when possible.
If insulin is used, combination therapy with a glucagon-like peptide 1 receptor agonist is recommended for greater efficacy and durability of treatment effect.
INDICATIONS FOR EARLY INITIATION OF INSULIN:
- Catabolic state (lean body, weight loss)
- HbA1c >10%
- RBS ≥300mg/dl
- Hospitalised patients
- Acute illness
Fig: algorithm for initiation and uptitration of Insulin therpay


SUGGESTED READINGS:
- American Diabetes Association;Standards of Medical Care in Diabetes—2022 Abridged for Primary Care Providers. Clin Diabetes 1 January 2022; 40 (1): 10–38.
- Fu Z, Gilbert ER, Liu D. Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr Diabetes Rev. 2013 Jan 1;9(1):25-53. PMID: 22974359; PMCID: PMC3934755.
- Blonde L, Umpierrez GE, Reddy SS, et al. American Association of Clinical Endocrinology Clinical Practice Guideline: Developing a Diabetes Mellitus Comprehensive Care Plan-2022 Update. Endocr Pract. 2022 Oct;28(10):923-1049. doi: 10.1016/j.eprac.2022.08.002. Epub 2022 Aug 11. PMID: 35963508.